
Case Report
Ann Hematol Oncol. 2017; 4(6): 1154.
Invasive Pulmonary Aspergillosis Complicated with Pneumopericardium and Pneumothorax in an Adult Patient with Acute Lymphoblastic Leukaemia, Successfully Treated with Amphotericin B: Case Report and a Review
Somawardana UABP¹*, Pieris DC², Gunasekara S³, Jayasekara PI4, Sigera LSM5 and Alagiyawanna AMALR6
¹Registrar in Haemato Oncology, National Cancer Institute, Sri Lanka
²Clinical Oncologist, National Cancer Institute, Sri Lanka
³Consultant Microbiologist, National Cancer Institute, Sri Lanka
4Consultant Mycologist, Department of Mycology, Medical Research Institute, Sri Lanka
5Department of Mycology, Medical Research Institute, Sri Lanka
6Registrar in Clinical Oncology, National Cancer Institute, Sri Lanka
*Corresponding author: Somawardana UABP, Senior Registrar in Haemato Oncology, National Cancer Institute, Maharagama, Sri Lanka
Received: April 14, 2017; Accepted: May 19, 2017; Published: June 05, 2017
Abstract
Invasive Pulmonary Aspergillosis (IPA) is a fatal opportunistic infection in immunocompromised patients which can be rarely associated with pleural or pericardial involvement. We describe a case of 30 year old male diagnosed with T-ALL who developed IPA complicated with pneumopericardium and pneumothorax during remission induction phase of chemotherapy, initially treated with voriconazole for 3 weeks, which caused severe hepatotoxicity and subsequently, successfully treated with IV amphorericin B for 8 weeks, in a resource limited setting. Cardiac tamponade was prevented by timely aspiration of the pericardial effusion.
Keywords: Aspergillosis; Pneumopericardium; Pneumothorax; Acute lymphoblastic leukaemia; Amphotericin B
Abbreviations
IA: Invasive Aspergillosis; CFR: Case Fatality Rate; IPA: Invasive Pulmonary Aspergillosis; ALL: Acute Lymphoblastic Leukemia; KOH: Potassium Hydroxide; HRCT: High Resolution Computed Tomography; CD: Cluster Differentiation
Introduction
Invasive aspergillosis (IA) is an almost fatal, difficult-totreat, opportunistic mould infection, most prevalent among immunocompromised hosts [1]. The overall case fatality rate (CFR) associated with IA is 58% according to a meta-analysis, although the mortality has significantly reduced over the past two decades [2].
Pneumopericardium is rare and it is caused by either the presence of a communication between the pericardial sac and an adjacent aircontaining organ, usually the lungs, resulting from trauma, fistula or iatrogenic causes; or by infection of the pericardium by gasforming microbes [3]. Invasive Pulmonary Aspergillosis (IPA) may be associated with haematogenous dissemination of the infection; however, involvement of the pleura or pericardium is extremely rare [4].
We are presenting a case of an adult Acute Lymphoblastic leukemia (ALL), who developed IPA during the remission induction phase of chemotherapy, complicated by pneumopericardium, pneumothorax and multiple cavitatory pulmonary lesions, initially treated with voriconazole which was subsequently omitted due to severe hepatotoxicity and later successfully treated with Amphotericin B. Given the rarity and challenges in management, particularly in a limited resource setting like ours, this case exemplifies treatment of the condition, while managing adverse events and prevention of cardiac tamponade with close monitoring and timely intervention.
Case Presentation
A 30 year old male presented with gross haematuria, bleeding from gums, fatigue, arthralgia, myalgia and fever for two weeks. At presentation his WBC count was 28.6x109/l, Haemoglobin 8.5 g/dl and platelets 3x109/l. On examination, multiple ecchymotic patches, multiple bilateral cervical and inguinal lymphadenopathy and palatal petichae were noted. Bone marrow aspiration and trephine biopsy with flowcytometry revealed a CD 10 positive T-ALL with 90% blasts.
UK ALL XII (version 4.1) chemotherapy protocol was started with platelet support. On day 17 sputum culture done for a productive cough revealed Aspergillus spp. and voriconazole was immediately started per oral. At this point, chest X-ray was normal and the blood cultures for bacteria and fungi were negative. Four days after completion of induction chemotherapy, patient developed intermittent fever which did not respond to broad spectrum antibiotic therapy according to the institutional protocol. On day 34, he also developed haemoptysis and a pleuritic type chest pain. Chest X-ray showed pneumopericardium and a right sided pneumothotax with 2 large cavitatory lesions in each hemithorax (Figure 1A and 1B).
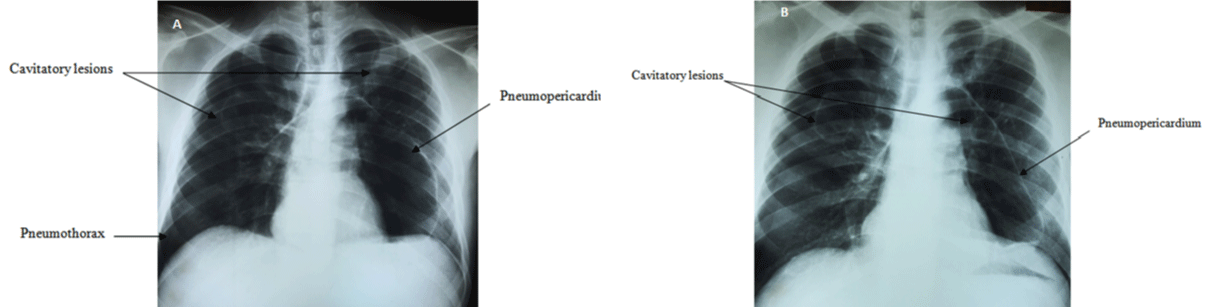
Figure 1A 1B: Chest X-ray PA showing massive pneumopericardium with a mild right sided pneumothorax and bilateral cavitatory lesions at two different
stages during the course.
A high resolution CT scan was performed which revealed the same with multiple cavities with necrotic material (Figure 2). Probable rupture of a cyst into the pericardium and pleura was suggested. No bronchopericardial fistula could be seen.
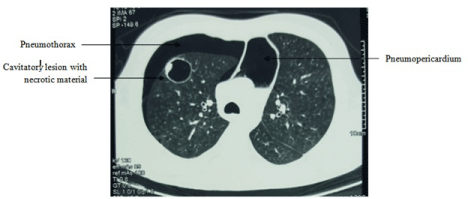
Figure 2: HRCT showing pneumopericardium with right sided pneumothorax
and a cavitatory lesion in the right upper lobe with necrotic material inside.
On 20 days (day 38) of treatment with voriconazole, he developed a severe transaminitis with SGOT and SGPT being more than 7 times the upper limit of normal ranges. The gamma glutamyltransferase levels were more than 10 times the upper limit. Therefore, voriconazole was replaced by Amphotericin. Bronchoalveolar lavage was not performed considering increased morbidity in a background of neutropenia.
The direct microscopic examination of sputum with 10% KOH demonstrated hyaline, septate fungal filaments branched dichotomously at acute angles. Fungal culture further revealed columnar conidial heads. The conidiophores were smooth walled, hyaline, uniseriate with closely compacted phialides only on upper portion of the vesicle. Figure 3 shows the microscopic view of the of the lacto phenol cotton blue mount of the colony. The species was identified as Aspergillus fumigatus according to these characteristic macroscopic and microscopic features.
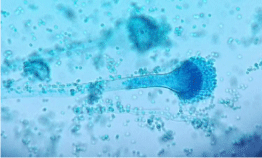
Figure 3: Microscopic view of the of the lacto phenol cotton blue mount of
the cultured colony.
On day 42, echocardiogram showed a pericardial effusion which was less than 1cm in thickness anteriorly and apically, 3 cm laterally and 2.5 cm posteriorly which was difficult to aspirate. Ejection fraction was 55% with preserved left ventricular systolic function, but a mild end diastolic right ventricular collapse was noted. Daily chest X-ray monitoring was done from this point.
Four days later, patient developed dyspnoea and chest X-ray revealed a massive pericardial effusion (Figure 4). Immediate cardiac intervention and aspiration was performed and the symptoms drastically improved. Pericardial fluid culture revealed no growth.
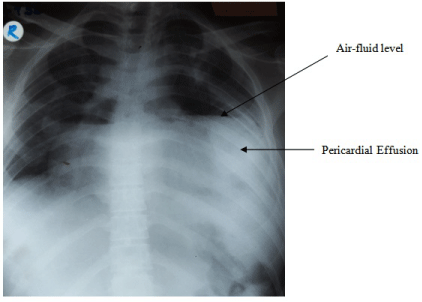
Figure 4: Chest X-ray PA showing massive pericardial effusion with an air
fluid level.
Amphotericin was continued for 8 weeks altogether until complete resolution of the cavitatory lesions, pneumopericardium and pneumothorax which was confirmed by a repeat X-ray (Figure 5) and HRCT. Although our patient developed persistent, moderate hypokalemia and hypomagnisemia due to Amphotericin, he was largely asymptomatic with regard to these adverse events. Electrolyte levels were monitored and corrected daily. Creatinine and blood urea nitrogen remained within normal limits and were monitored twice a week. He also developed febrile episodes in relation to the Amphotericin infusions which were symptomatically managed.
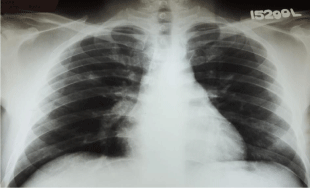
Figure 5: Chest X-ray PA done at the end of the treatment showing complete
resolution of the abnormalities.
A bone marrow biopsy confirmed morphologic remission. Phase II induction chemotherapy, which was delayed by 5 weeks, was restarted, while he was on amphotericin, when the counts recovered and the sputum cultures were all negative for fungi and bacteria. Currently the patient is on maintenance chemotherapy with no further complications.
Discussion
Although pneumopericardium is reported as a rare complication of thoracic trauma, approximately 25% can be associated with disease in the contiguous organs, commonly the lungs. However, involvement of the pleura or pericardium due to IPA is extremely rare [4]. There are only seven reported cases in the English-language literature which demonstrates the association between IPA and pneumopericardium among leukaemic patients, and the Table 1 summarizes these cases including the presenting case [3,5-13]. Two more cases have also been reported which demonstrates the same clinical association, but the underlying conditions were immunodeficiency and organ transplantation [14,15].
First Author
Year of Publication
Age of patient (years)
Type of underlying leukaemia
Antifungal/s used
Duration of therapy (weeks)
Müller [8]
1987
40
CML
A
N/A
Owens [9]
1990
14
ALL
A
6
Van Ede [10]
1994
29
AML
A+I
N/A
Merino [11]
1995
7
ALL
A+I
4
Ödev [3]
2002
14
ALL
A
N/A
Yilmaz [12]
2007
59
ALL
A+C
N/A
Alviar [13]
2014
47
ALL
A+V
12
Somawardanaª
2017
30
ALL
V?A
11
Table 1: Summary of leukaemia cases reported with IPA and pneumopericardium.
All cases, except one, involved men, with an underlying diagnosis of leukemia, with similar clinical presentations of pleuritic chest pain, dyspnea and haemoptysis. Only one case was reported in the post transplant setting. Most cases identified A. fumigatus while only one study showed A. niger in the pericardial fluid. All cases were treated with amphotericin B, however, in 4 cases, combination therapy with itaconazole, voriconazole or caspofungin was used. 3 out of 8 cases were successfully treated while others succumbed due to disease.
The vast majority of IPA is seen among patients with haematological malignancies particularly during the remission induction of ALL and this has emerged as a major challenge in the management of these malignancies [5,6]. The major risk factors for IPA in this group of patients include prolonged neutropenia, prolonged, high dose corticosteroid therapy, leukaemia, intensive chemotherapy, and were all present in our patient predisposing to IPA [6,7].
Intravenous or oral voriconazole is recommended for primary treatment of IPA [5]. Voriconazole was compared with amphotericin B in a large prospective controlled clinical trial which showed that the former significantly improved the response rate and survival at 12 weeks [16]. It was shown to have a better pericardial penetration compared to other drugs [17]. Further, it was also shown to be more cost effective compared to amphotericin B with a 10% lower overall treatment cost per patient [18]. Although severe hepatotoxicity is rare, this is unpredictable since there is inter-individual and intra-individual variability in voriconazole, plasma levels [7]. Oral voriconazole caused severe heptotoxicity in our patient which compelled us for its discontinuation. Non-Indian Asian populations like ours have a higher frequency of single-nucleotide polymorphisms of CYP2C19 contributing to slow metabolism of voriconazole, possibly resulting in significant variation in plasma concentrations [5]. This may be the reason for intolerance of this drug in our patient.
Amphotericn B induced nephrotoxicity is characterized by azotemia, hypokalemia, hypomagnisemia and renal tubular acidosis [19]. Our patient persistently had hypokalemia and hypomagnisemia which were monitored and corrected regularly. However, creatinine and blood urea nitrogen remained within normal limits. Infusion related side effects were symptomatically dealt with. We also made sure no other nephrotoxic drugs are given concurrently.
Lipid based preparations of amphotericin B such as liposomal form is associated with less nephrotoxicity and infusion related side effects [20]. However, in a free health system like ours, the cost effectiveness of this drug is questionable and a continuous supply cannot be assured as the drug is required for long periods.
Duration of antifungal treatment for IPA is not clearly defined. It is recommended that treatment be continued at least for 6-12 weeks; in immunocompromised patients, treatment should be continued throughout the period of immunosuppression and until lesions have resolved [5]. We administered voriconazole for 24 days which was replaced by amphotericin B, and the total duration of antifungal therapy was 11 weeks. We stopped the treatment on confirmation of resolution of pulmonary lesions.
References
- Thammahong A, Thayidathara P, Suksawat K, Chindamporn A. Invasive Aspergillus Infections in a Thai Tertiary-Care Hospital during 2006-2011. Advances in Microbiology 2015: 5; 298-306.
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001; 32: 358-366.
- Ödev K, Caliskan U, Emlik D, Koc H, Koc S. Pneumomediastinum and pneumopericardium due to intracavitary aspergilloma: an unusual complication of fungal pneumonia. Pediatr Radiol. 2002; 32: 143-145.
- Walsh TJ, Bulkley BH. Aspergillus pericarditis: clinical and pathologic features in the immunocompromised patient. Cancer. 1982; 49: 48-54.
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, et al. Treatment of Aspergillosis: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin Infec Dis 2008; 46: 327-360.
- Herbert PA, Bayer AS. Fungal pneumonia. Invasive pulmonary aspergillosis. Review. Chest 1981; 80: 220-225.
- M Kousha, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011; 20: 156-174.
- Müller NL, Miller RR, Ostrow DN, Nelems B, Vickars LM. Tension pneumopericardium: an unusual manifestation of invasive pulmonary aspergillosis. AJR Am J Roentgenol. 1987; 148: 678-680.
- Owens CM, Hamon MD, Graham TR, Wood AJ, Newland AC. Bronchopericardial fistula and pneumopericardium complicating invasive pulmonary aspergillosis. Clin Lab Haematol. 1990; 12: 351-354.
- van Ede AE, Meis JF, Koot RA, Heystraten FM, de Pauw BE. Pneumopericardium complicating invasive pulmonary aspergillosis: case report and review. Infection. 1994; 22: 102-105.
- Merino JM, Diaz MA, Ramirez M, Ruano D, Madero L. Complicated pulmonary aspergillosis with pneumothorax and pneumopericardium in a child with acute lymphoblastic leukemia. Pediatr Hematol Oncol. 1995; 12: 195-199.
- Yilmaz M, Demirel AE, Izmir S, Soysal T, Mert A. Pneumopericardium due to invasive pulmonary aspergillosis. J Infect Chemother. 2007; 13: 341-342.
- Alviar CL, Doherty B, Vaduganathan M. Acute lymphocytic leukemia with superimposed invasive aspergillosis and pneumopericardium successfully treated with voriconazole. Proc (Bayl Univ Med Cent) 2014; 27: 250-252.
- Serrano-Gonzalez A, Merino-Arribas JM, Ruiz-Lopez MJ, Casado-Flores J. Invasive pulmonary aspergillosis with pneumopericardium and pneumothorax. Pediatr Radiol. 1992; 22: 601-602.
- Sengul S, Keven K, Kutlay S, Ergun I, Azap A, Erbay B. Quiz page. Invasive pulmonary aspergillosis presenting with a pulmonary nodule, pneumomediastinum, pneumothorax, and pneumopericardium. Am J Kidney Dis. 2004; 44: A47, E47-9.
- Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann JW, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347: 408-415.
- Poupelin JC, Philit F, Richard JC, Badet M, Lemasson S, Bayle F, et al. Pericardial and pleural diff usion of voriconazole during disseminated invasive aspergillosis: report of a case with successful outcome. Intensive Care Med. 2006; 32: 939-940.
- Wenzel R, , Del Favero A, Kibbler C, Rogers T, Rotstein C, Mauskopf J, et al. Economic evaluation of voriconazole compared with conventional amphotericin B for the primary treatment of aspergillosis in immunocompromised patients. J Antimicrob Chemother. 2005; 55: 352-361.
- Laniado-Laborín R, Cabrales-Vargas MN. Amphotericin B: side effects and toxicity. Rev Iberoam Micol. 2009; 26: 223-227.
- Cagnoni PJ, Liposomal amphotericin B versus conventional amphotericin B in the empirical treatment of persistently febrile neutropenic patients. J Antimicrob Chemother. 2002; 49: 81-86.
Citation:Somawardana UABP, Pieris DC, Gunasekara S, Jayasekara PI, Sigera LSM and Alagiyawanna AMALR. Invasive Pulmonary Aspergillosis Complicated with Pneumopericardium and Pneumothorax in an Adult Patient with Acute Lymphoblastic Leukaemia, Successfully Treated with Amphotericin B: Case Report and a Review. Ann Hematol Oncol. 2017; 4(6): 1154. ISSN:2375-7965