
Special Article - Multiple Myeloma
Ann Hematol Oncol. 2017; 4(10): 1174.
Blinatumomab Induced Response of Multiply Refractory Multiple Myeloma in the Context of Secondary Pre-B Cell Acute Lymphoblastic Leukemia
Pratz KW*, Gojo I, Gocke C, Matsui W and Huff CA
Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, USA
*Corresponding author: Keith W. Pratz, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins University, CRB1 Room 2M45, 1650 Orleans St, Baltimore MD, 21231, USA
Received: August 30, 2017; Accepted: October 06, 2017; Published: October 24, 2017
Abstract
We report the case of a 70 year old female who developed Pre-B Acute Lymphoblastic Leukemia (ALL) while undergoing lenalidomide therapy for multiple myeloma (MM). Molecular studies of the ALL revealed hypodiploid karyotype with distinct genetic changes other than those seen in prior myeloma samples. She underwent cytoreductive therapy with prednisone and cyclophosphamide and then received induction with blinatumomab. After her first cycle, she achieved a MRD negative complete remission of her ALL and a deepening of her MM remission to a VGPR by IMWG criteria. She went on to get a second cycle of blinatumomab with continued complete remission of her ALL, normalization of complete blood count and an ongoing VGPR of her MM. Laboratory studies have established the myeloma stem cell is CD19 positive suggesting a potential mechanism of action of improvement of her myeloma in this case.
Keywords: Multiple myeloma; Blinatumomab; Acute lymphoblastic leukemia
Introduction
The biology of Multiple Myeloma and Pre-B ALL, both B-Cell diseases, has revealed the presence of CD19 in tumor initiating cells [1,2]. Blinatumomab is a bi-specific antibody construct developed with binding domains for both CD19 and CD3 and is approved for relapsed or refractory B-cell ALL with remarkable rates of complete remission in highly refractory cases [3]. We report here the case of a 70-year-old who has been under therapy for Multiple Myeloma for 8 years with various multi-agent regimens and was in a partial remission on single agent lenalidomide, when she developed pre-B ALL. Lenalidomide has been suggested to play a role in the development of secondary ALL in the context of myeloma therapy [4]. We elected to treat her with blinatumomab after deeming her unfit for conventional ALL induction therapy and definitive management with allogeneic transplant due to age and comorbidities. She achieved a complete remission of her ALL and a VGPR of her MM with normalization of her CBC, and no evidence of MRD by Flow for ALL.
Case Presentation
The patient was diagnosed with ISS Stage I multiple myeloma in November 2008 when blood work revealed hypercalcemia (Ca 12.2) and a monoclonal protein (IgG kappa 4.4 gm/dL M spike). On presentation multiple lytic lesions and hemoglobin of 10.1 were noted. Myeloma FISH panel revealed +3, +9, + 15, +11 consistent with hyperdiploid phenotype with no del 13Q, del 17p or IgH translocations. Bone marrow examination revealed 30% plasma cells and Flow cytometry of the diagnostic specimen revealed CD38 bright, CD20 negative, CD19 positive (Dim), surface kappa light chain dim, cytosolic kappa light chain bright abnormal plasma cells. She was initially treated with bortezomib and dexamethasone for 3 cycles resulting in stable disease. Therapy was changed to lenalidomide (len)/dexamethasone (dex) in 3/09 for one cycle resulting in a partial remission. Len/dex was then discontinued due to acute renal insufficiency and she remained off all therapy for 6 months before enrolling on a research protocol with BMS-833923 to which she initially had stable disease followed by progression after 5 cycles of therapy. She was observed off therapy for 6 months and then treated with cyclophosphamide / bortezomib / dexamethasone for 4 cycles resulting in a minor response. Again, she remained off all therapy for almost two years before starting single agent Lenalidomide in May 2013, initially 15 mg a day x 21 out of every 28 days achieving a partial remission and then increased to 25 mg a day x 21 out of every 28 days in March 2015 due to evidence of early progression. In August 2016, her M-Spike measured 0.47 g/dL with Kappa free light chains of 50.2 mg/L. CBC at the time revealed Hb of 11.6, WBC of 2.15 and platelets of 60K and she met criteria for a PR.
In September 2016 she developed fevers, night sweats, and dropping platelets of 20K, WBC of 3.3 with 78% lymphocytes and hemoglobin down to 10.0 g/dL. Her lenalidomide was discontinued. Bone marrow evaluation revealed Pre-B Cell Acute Lymphoblastic Leukemia (ALL) with 95% blasts with immunophenotype via Flow cytometry staining CD 38+(moderate), CD19+(heterogeneous), CD10+, CD34+, surface and cytoplasmic Kappa negative, surface and cytoplasmic Lambda negative, CD38 moderate.
Her molecular studies of the B-Cell ALL revealed hypodiploid karyotype (33,XX,-2,-3,-4,-5,-7,-9,-11,-12,-13,-15,-16,-17,- 20[4]/33,sl,add(12)(q24.1),+mar[6]/66,slx2[2]). Fish studies revealed -5, -7, -11, -20, and subset with extra copies of chromosome 8 (35% trisomy/tetrasomy).
She was treated with cyclophosphamide 200 mg/m2 along with 60 mg/m2 of prednisone for 5 days for cytoreduction. She received one dose of methotrexate intrathecally day 3 of cyclophosphamide. She was then initiated on blinatumomab 9 mcg per day, days 1-7, then 28 mcg/day, days 8-28. Dexamethasone 20 mg was given prior to blinatumomab day 1 and at dose escalation on day 8. She tolerated blinatumomab well and restaging bone marrow on count recovery revealed a complete remission. MRD assessment via FLOW for B-Cell ALL was negative. Repeat fish studies were negative for both ALL and myeloma associated changes. Serologic studies showed improvement, but persistence, of a monoclonal spike measuring 0.22 g/dL with normalization of serum free light chains (Kappa 8.6 mg/L, Lambda 7.5 mg/L, K/L ratio 1.15) (Figure 1). She went on to receive a second cycle of blinatumomab and on recovery a repeat bone marrow biopsy confirmed ongoing complete remission with no evidence of B-Cell ALL by FLOW to a MRD negative level of 0.01% and no immunophenotypic evidence of myeloma by FLOW or core bone marrow staining. Monoclonal protein was reassessed after cycle 2 of Blinatumomab and was found to be present at .13 g/ dL and IFE confirmed this was consistent with her IgG Kappa seen previously. Her CBC normalized for the first time since her diagnosis of myeloma. She continues to have a small but measurable M-Spike (0.08 g/dL) 322 days post last dose of Blinatumomab without other therapy with normal free light chains (Kappa 8.1 mg/L, Lambda 5.7 mg/L Ratio 1.42).
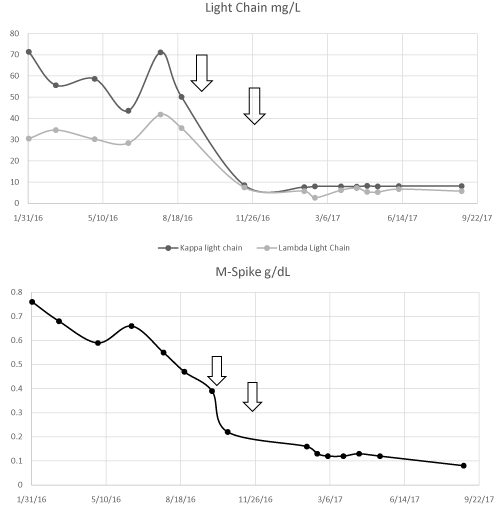
Figure 1: Monoclonal Protein response. Arrow indicates courses of
blinatumomab therapy.
Discussion
We report the first use of the CD19/CD3 bi-specific T-cell engager blinatumomab in a patient with active multiple myeloma. This patient was able to achieve a MRD negative remission in her B-Cell ALL and VGPR of her Multiple Myeloma. Of note, the initial immunophenotyping of the myeloma in this case did reveal positive staining for CD19 which is somewhat atypical for myeloma bulk tumor. While it’s possible the cyclophosphamide and dexamethasone could have played a role in the response here, this patient had been extensively treated with those agents in the past and never has achieved this level of monoclonal protein reduction. This case may reveal a role for CD19 directed therapies in Multiple Myeloma. The tumor initiating cell for myeloma has been described through serial transplant studies to be CD19 positive and resembling the memory B-Cell [1]. Strategies to target this myeloma stem cell are needed as clonogenic studies of these cells reveal resistance to conventional myeloma agents such as bortezomib, lenalidomide, cyclophosphamide and dexamethasone [1]. We feel the exploration of blinatumomab as myeloma directed therapy warrants further clinical evaluation based not only on this case but on the biologic rationale noted above.
References
- Matsui W, Wang Q, Barber JP, Brennan S, Smith BD, Borrello I, et al. Clonogenic multiple myeloma progenitors, stem cell properties, and drug resistance. Cancer Res. 2008; 68: 190-197.
- Rehe K, Wilson K, Bomken S, Williamson D, Irving J, den Boer ML, et al. Acute B lymphoblastic leukaemia-propagating cells are present at high frequency in diverse lymphoblast populations. EMBO Mol Med. 2013; 5: 38-51.
- Topp MS, Gökbuget N, Stein AS, Zugmaier G, O’Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, singlearm, phase 2 study. Lancet Oncol. 2015; 16: 57-66.
- Tan M, Fong R, Lo M, Young R. Lenalidomide and secondary acute lymphoblastic leukemia: a case series. Hematol Oncol. 2015.