
Case Report
Ann Hematol Oncol. 2017; 4(12): 1183.
Hodgkin Lymphoma Presenting with Dural Involvement
Voshtina E, Olteanu H, Graner B and Shah N*
Froedtert & Medical College of Wisconsin, USA
*Corresponding author: Shah N, The Medical College of Wisconsin, USA
Received: October 19, 2017; Accepted: November 16, 2017; Published: December 07, 2017
Abstract
Hodgkin Lymphoma (HL) is a B-cell neoplasm that rarely presents with central nervous system (CNS) extranodal manifestation. This presentation can occur as relapsed disease as well as at initial diagnosis. In this report, we present a patient with dural involvement of HL. Systemic chemotherapy with ABVD (doxorubicin, bleomycin, vinblastine, and decarbazine) in addition to intrathecal therapy (IT) with intrathecal chemotherapy may be a treatment option for patients with isolated dural involvement with CNS HL. There is no current consensus for treatment modality of CNS HL, however, our patient with dural disease was successfully treated with ABVD systemic chemotherapy and IT MTX and ARA-C and is now >12 months in an ongoing remission. The role of CNS penetrating agents for isolated dural involvement is unclear given it is not protected by the blood brain barrier (BBB). As evidenced by this case with appropriate therapy patients can achieve complete remission (CR) in CNS HL.
Keywords: Hodgkin lymphoma; Central nervous system; Doxorubicin, bleomycin, vinblastine, and decarbazine
Introduction
Hodgkin Lymphoma (HL) is a B-cell neoplasm that typically presents with enlarged lymph nodes and “B” symptoms. Many patients at presentation will have advanced disease and some will develop extranodal lesions. Central nervous system (CNS) involvement is an extremely rare extranodal manifestation of HL occurring at a frequency of 0.02-0.7% in patients [1-3]. Although rare, there have been several cases describing CNS HL [4-6]. The current knowledge of CNS HL and treatment approach is mostly derived from these case reports. Treatment has often included radiation, systemic chemotherapy, surgical resection, and combined modality therapy; however, no consensus has been reached about the best treatment option. Despite aggressive treatments, overall prognosis in patients with CNS HL is poor. In our case, we describe a patient with HL presenting with dural involvement.
Case Presentation
A 38 year old male presented with a 5 week history of new onset headache. The patient underwent a magnetic resonance imaging (MRI) brain scan which demonstrated dural thickening, and enhancement along the anterior falx and the right tentorium (Figure 1A and D). Imaging was concerning for an infectious, inflammatory, or neoplastic process. He had a lumbar puncture, which was negative for infection and malignancy by flow cytometry and cytology. A bone marrow biopsy demonstrated hypocellularity with normal karyotype with no evidence of malignancy. The patient then underwent a computed tomography (CT) neck/chest/abdomen/pelvis and positron emission tomography (PET) scan, which demonstrated a single enlarged FDG-avid supraclavicular node (Figure 1G). The node was excised, and pathology demonstrated a lymph node with effaced architecture and nodules surrounded by thick collagen bands. These nodules were comprised of small lymphocytes with interspersed large mono- or binucleated Reed-Sternberg cells with prominent eosinophilic nucleoli and abundant light eosinophilic cytoplasm. There was focal retraction artifact present around the large cells, as well as scattered “mummified” cells. The Reed-Sternberg cells were CD15+ and CD30+, weakly PAX-5+, CD3-, CD20-, and CD45- . Background lymphocytes were composed of CD3+ T-cells and CD20+ B-cells. The excised lymph node pathology was diagnostic for nodular sclerosis classical HL (Figure 2). The patient was referred to oncology.
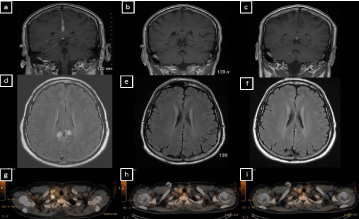
Figure 1: Pretreatment and posttreatment CT scans and PET scans.
While awaiting his oncology appointment, the patient developed progressive headaches with associated diplopia along with blurry vision in his left eye. An ophthalmologic evaluation was negative for lymphomatous involvement of the globe or optic nerve. Repeat imaging of the brain again showed an enhancing dural lesion along the anterior falx and the right tentorium. A dural biopsy was considered, but given the location, it was felt a biopsy would be high risk and potentially low yield. The patient underwent two additional lumbar punctures, both of which were negative for an infectious or neoplastic etiology with a normal angiotensin converting enzyme level. He had an octreotide scan which was equivocal, but demonstrated nonspecific increased activity along the right tentorium and midline falx. With the patient’s extensive work up with multiple lumbar punctures returning negative for infectious or inflammatory etiology, it was felt that in the setting of supraclavicular lymph node pathology revealing HL, MRI imaging findings were most consistent with metastatic HL involvement of the dura.
The patient was started on dexamethasone with improvement in his headache within 24 hours of initiation. He then started treatment with ABVD chemotherapy (doxorubicin, bleomycin, vinblastine, and decarbazine) in addition to intrathecal therapy (IT) alternating IT methotrexate (MTX) and IT cytarabine (ARA-C) with each cycle. Interim PET/CT after 2 months of ABVD demonstrated a systemic complete metabolic response (Figure 1H). He tolerated treatment well and had improvement in his diplopia and blurry vision. At the end of treatment, he had complete resolution of presenting symptoms of headache and blurry vision. Interim and end of treatment MRI brain demonstrated resolving dural disease (Figure 1B, C, E and F). End of treatment PET/CT remained negative and the patient was deemed to be in complete remission (CR) from his HL (Figure 2). He is now 12 months post-treatment with no signs of relapsed disease.
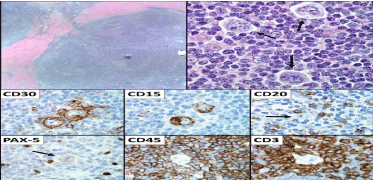
Figure 2: Excised lymph node pathology demonstrating nodular sclerosis classical HL
Discussion
CNS involvement in HL is extremely rare and estimated at 0.02-0.7% [1-3]. Presenting sites of involvement, have included most commonly brain parenchyma (64%); dura and leptomeninges (19%); corpus callosum (3%); and pituitary (3%) [7-8]. Based on site of involvement, initial neurological presentation has most commonly included cranial nerve palsies (55%), headaches (36%), and paresis (33%) [7]. CNS involvement can present as a primary manifestation or dissemination of systemic disease, however, CNS HL without any systemic involvement is considered even rarer [9-11]. CNS involvement of HL that occurs at relapse has been reported to have poorer prognosis, in contrast to CNS HL that presents at diagnosis [3,8]. The most common concurrent areas of disease when the CNS is involved are lymph nodes, bone marrow, and lungs [2]. Three forms of dissemination to the CNS have been described and include hematogenous spread from nodal sites, direct extension via the skull or dura, and meningeal metastasis [12,13]. The predominant histological subtype is reported to be nodular sclerosis in patients with CNS HL, followed by mixed cellularity and then lymphocyte predominant [2,3]. No risk factors for development of CNS HL have been recognized, however, concurrent EBV infection or immunosuppression have been suggested as predisposing factors [14].
While the current standard of care of advanced stage HL without CNS involvement is ABVD chemotherapy for 6 cycles, the optimal treatment modality for CNS HL is unknown due to its rarity and has included surgery, radiation, chemotherapy, or combined modalities [15]. The general approach to lymphomatous involvement in the CNS includes either systemic or IT chemotherapy and/or radiation therapy. More specifically, high dosages of systemic MTX ( >3000 mg/ m2) are felt to have adequate CNS penetration and this is currently the accepted standard for patients with high risk of CNS involvement or those with concurrent CNS involvement in non-Hodgkin lymphoma [16]. Given the rarity of CNS involvement by HL, a multitude of approaches including high dose MTX, high dose ARA-C, and radiation therapy have been utilized [3]. Despite treatment, patients with CNS involvement of HL are felt to have a worse prognosis. In a multi-center review by Cheah, et al. after varying treatments, the overall response rate in patients with CNS HL was 65% but only a quarter of patients had prolonged disease free survival. Response rates were found to be highest among those patients that received radiation therapy or systemic chemotherapy [3]. Another study showed radiographic response after use of varied treatments in 69% of patients and complete remission in 56% [8].
For patients with dural disease alone, it is unclear whether CNS penetrating agents are indicated as the vasculature for the dura is not felt to be protected by the blood brain barrier (BBB) [17]. In patients with primary dural lymphoma, systemic chemotherapy alone has been given without CNS penetrating drugs with excellent outcomes [17]. Given that ABVD is the proven standard of care for HL, for this patient we treated with a combination of ABVD + IT chemotherapy every 2 weeks. With initiation of treatment, the patient had rapid resolution of symptoms which correlated with MRI imaging. While a biopsy confirmation was not able to be performed in this case, the clinical-radiographic impression along with treatment response best fit a diagnosis of HL.
References
- Re D, Fuchs M, Schober T, Engert A, Diehl V. CNS Involvement in Hodgkin’s Lymphoma. J Clin Oncol. 2007; 25: 3182.
- Sapozink MD, Kaplan HS. Intracranial Hodgkin’s disease. A report of 12 cases and review of the literature. Cancer. 1983; 52: 1301-1307.
- Cheah CY, Bröckelmann PJ, Chihara D, Moskowitz AJ, Engert A, Jerkeman M, et al. Clinical characteristics and outcomes of patients with Hodgkin lymphoma with central nervous system involvement: An international multicenter collaboration. Am J Hematol 2016; 91: 894-899.
- Akyüz C, Yalcin B, Atahan IL, Varan A, Kutluk MT, Büyükpamukçu M. Intracranial involvement in hodgkin’s disease. Pediatr Hematol Oncol. 2005; 22: 589-596.
- Van Blydenstein SA, Patel M, Philip V, Lakha A, Pather S, Westgarth-Taylor T, et al. Classical Hodgkin Lymphoma involving the central nervous system (brain) - an unusual presentation. Clin case reports. 2014; 2: 88-92.
- Morawa E, Ragam A, Sirota R, Nabhan C. Hodgkin’s lymphoma involving the CNS. J Clin Oncol 2007; 25: 1437-1438.
- Hirmiz K, Foyle A, Wilke D, Burrell S, Brownstone R, Ago C, et al. Intracranial presentation of systemic hodgkin’s disease. Leuk Lymphoma. 2004; 45: 1667-1671.
- Gerstner ER, Abrey LE, Schiff D, Ferreri AJM, Lister A, Montoto S, et al. CNS Hodgkin lymphoma. Blood. 2008; 112: 1658-1661.
- Gallamini A, Hutchings M, Ramadan S. Clinical presentation and staging of Hodgkin lymphoma. Semin Hematol. 2016; 53: 148-154.
- Guermazi A, Brice P, de Kerviler E, Fermé C, Hennequin C, Meignin V, et al. Extranodal Hodgkin Disease: Spectrum of Disease. RadioGraphics. 2001; 21: 161-179.
- Elwell VA, Carney L, Johns P, Grieve JP. Dural infiltration of metastatic Hodgkin’s lymphoma. Br J Neurosurg. 2008; 22: 439-440.
- Gharbaran R, Park J, Kim C, Goy A, Suh KS. Circulating tumor cells in Hodgkin’s lymphoma-A review of the spread of HL tumor cells or their putative precursors by lymphatic and hematogenous means, and their prognostic significance. Crit Rev Oncol Hematol. 2014; 89: 404-417.
- Grimm S, Chamberlain M. Hodgkin’s Lymphoma: A Review of Neurologic Complications. Adv Hematol. 2011; 2011: 624578.
- Alexander FE, Jarrett RF, Lawrence D, Armstrong AA, Freeland J, Gokhale DA, et al. Risk factors for Hodgkin’s disease by Epstein-Barr virus (EBV) status: prior infection by EBV and other agents. Br J Cancer. 2000; 82: 1117- 1121.
- Vassilakopoulos TP, Johnson PWM. Treatment of advanced-stage Hodgkin lymphoma. Semin Hematol. 2016; 53: 171-179.
- Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, Takvorian T, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010; 116: 4283-4290.
- Matmati K, Matmati N, Hannun YA, Rumboldt Z, Patel S, Lazarchick J, et al. Dural MALT lymphoma with disseminated disease. Hematol Rep. 2010; 2: e10.