
Special Article - Leukemia
Ann Hematol Oncol. 2018; 5(1): 1188.
Feasibility and Safety of Cardiopulmonary Exercise Testing in Acute Leukemia Patients during Induction Chemotherapy
Coffman EM¹, Bryant AL², Deal AM³, Wang Y4, Hanson ED5, Phillips B6, Foster M7, Wood WA7, Bailey C8, Muss H9 and Battaglini CL10*
¹Clinical Research Coordinator, The University of North Carolina at Chapel Hill, USA
²School of Nursing,The University of North Carolina at Chapel Hill, USA
³Biostatistics Core Facility, The University of North Carolina at Chapel Hill, Lineberger Comprehensive, USA
4Department of Biostatistics, The University of North Carolina at Chapel Hill, Lineberger Comprehensive Cancer Center, USA
5Department of Exercise and Sport Science, University of North Carolina at Chapel Hill, USA
6Clinical Research Coordinator, Hemophilia and Thrombosis Center, The University of North Carolina at Chapel Hill, USA
7Division of Hematology/Oncology, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill, USA
8Clinical Research Specialist I, Survivorship, Lifestyle, and Supportive Health, AHSP-Samuel Oschin, Comprehensive Cancer Institute, USA
9Geriatric Oncology Program, Lineberger Comprehensive Cancer Center, The University of North Carolina at Chapel Hill
10Department of Exercise Sports and Science, Lineberger Comprehensive Cancer Center, UNC Exercise Oncology Research Laboratory, The University of North Carolina at Chapel Hill, USA
*Corresponding author: Battaglini C, Department of Exercise Sports and Science, Lineberger Comprehensive Cancer Center, UNC Exercise Oncology Research Laboratory, University of North Carolina at Chapel Hill, USA
Received: November 20, 2017; Accepted: January 18, 2018; Published: February 05, 2018
Abstract
The interest and use of exercise as an adjuvant therapy for the alleviation of treatment-related side effects in cancer patients has grown significantly the past decade. The need for the use of objective measures to not only inform the prescription of exercise, to assess the beneficial effects of exercise, but also as a screening tool for exercise participation is paramount for the development of evidence-based exercise programs for cancer patients.
Purpose: The purpose of this study was to evaluate the feasibility and safety of administering a cardiopulmonary exercise test (CPET) in newly diagnosed acute leukemia patients enrolled in a randomized clinical trial examining the effects of an exercise program during induction chemotherapy.
Methods: Seventeen patients recently diagnosed with acute leukemia were randomized into the exercise intervention (n=8) or usual care (n=9). Patients attempted a multistage CPET at the hematology oncology unit to assess peak oxygen uptake (VO2peak) at baseline (within 4 days of admission for induction chemotherapy) and at the end of the exercise program (at discharge from hospital).
Results: Only 47% of all planned baseline and post-intervention CPETs (total of 16 tests out of 34) were completed. Exploratory analyses revealed a significant correlation between VO2peak and the Timed Up and Go test (TUG, r=-0.6, p=.003) and 6-minute walk distance test (6MWT, r=0.61, p=.001, respectively).
Conclusion: The administration of a CPET in newly diagnosed acute leukemia participating in an exercise study during induction therapy appears to be safe, however, based on the feasibility criteria adopted in this study, the use a CPET for the assessment of cardiopulmonary function (CRF) at baseline and discharge does not appear to be feasible, since the large majority of the patients enrolled in the control group were not able to complete the CPET at discharge.
Keywords: Acute leukemia; Acute myelogenous leukemia; Hematological cancers
Introduction
Acute leukemia is a hematological cancer that often requires immediate hospitalization for initiation of induction chemotherapy due to its rapid onset and development. In 2017, it is estimated there will be 62,130 new cases of leukemia. Even though relative survival rate has increased since the 1960s, in patients diagnosed with acute myelogenous leukemia (AML) for example, the fiveyear overall relative survival rates remain low at 26%, compared to other types of hematological cancers [1]. Initial treatment (induction chemotherapy), consists of daily high-dose of chemotherapy administration for a week, followed by 3-6 weeks of inpatient recovery. The aggressive nature of treatment puts these patients at greater risk for serious treatment-associated complications including nausea, vomiting, and diarrhea. The burden of these symptoms can result in poor nutrition and reduced physical activity. The physical and psychological effects of the high-dose chemotherapy and recovery are the perfect recipe for reduced physical functioning, significant reductions in Cardiorespiratory Fitness (CRF) and potentially less favorable treatment prognosis and poor quality of life [2].
Cardiorespiratory fitness (CRF) has been shown to be predictive of mortality in healthy adults, those with chronic disease (i.e. cardiovascular disease [3-5], and most recently, individuals with certain types of cancers [6,7]. Cardiopulmonary exercise testing (CPET), is considered the gold standard method for the evaluation of CRF. Guidelines and recommendations for the assessment of CRF in cancer patients have been previously published [8]. However, issues associated with the administration of symptom-limited CPET in inpatient cancer populations include but are not limited to: accessibility to a metabolic cart, personnel qualified to conduct such tests, time constraints with treatments, and most importantly, the ability and willingness of patients to undergo such testing during different phases of treatment.
Few studies have attempted to use exercise interventions aimed to alleviate the severe physical function decline observed during acute leukemia treatment. However, the existing exercise trials that have attempted to alleviate treatment-related side effects of chemotherapy have yielded promising results [9-13]. Even though most of these progressive and innovative trials used objective measurements for the assessment of different fitness and functionality parameters, none have directly measured CRF via gold standard CPET with indirect calorimetry.
The purpose of this study was to evaluate the feasibility and safety of administering a CPET in recently diagnosed acute leukemia patients enrolled in a randomized clinical trial examining the effects of an exercise program during induction chemotherapy. Exploratory analyses were used to examine the associations between changes in CRF and physical function measures.
Methods
General procedures
This randomized clinical trial (NCT 02246907) recruited patients between October 2014 and November 2015. Adults with acute leukemia were recruited at the NC Cancer Hospital (Lineberger Comprehensive Cancer Center) within 4 days of admission for induction treatment. If a patient demonstrated interest in participating, their oncologists were consulted and asked to evaluate the patient’s eligibility to enroll in the study based on the inclusion/ exclusion criteria. Inclusion criteria consisted of: 1) adults > 18 years old, newly diagnosed with AML or ALL, 2) admitted to begin induction chemotherapy with an expected hospital stay of 4-6 weeks, and 3) able to speak and understand English. Exclusion criteria included: cardiovascular disease; acute or chronic respiratory disease; acute or chronic bone, muscle or joint abnormalities; altered mental state, dementia, or any other psychological condition that would prevent understanding of informed consent, being actively treated for other co-morbidities that compromise safe participation in a maximal/peak cardiopulmonary exercise testing (CPET). If a patient met the inclusion/exclusion criteria, and after the oncologist cleared the patient to enroll in the study, patients were introduced to the study. Those interested in participating in the study were asked to sign informed consent approved by the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center Protocol Review Board and the UNC Biomedical Institutional Review Board prior to participating in any study activities.
Eighty-two patients were screened for participation in the study (Figure 1). Sixty-four failed to meet the inclusion criteria: cardiac, respiratory, joint/musculoskeletal comorbidities (n=24), diagnosed with another type of hematological cancer besides leukemia (n=12), missed recruitment window (n=10), a hospital stay less than 3 weeks (n=5), less than 18 years old (n=3), exercise physiologist not available for testing (n=3), unable to speak or understand English (n=2), VO2peak testing equipment unavailable (n=2), bleeding, thrombosis, hemodynamically unstable, uncontrolled pain (n=2), prior malignancy (n=2), unable to provide informed consent (n=1), or patient discharged to hospice (n=1).
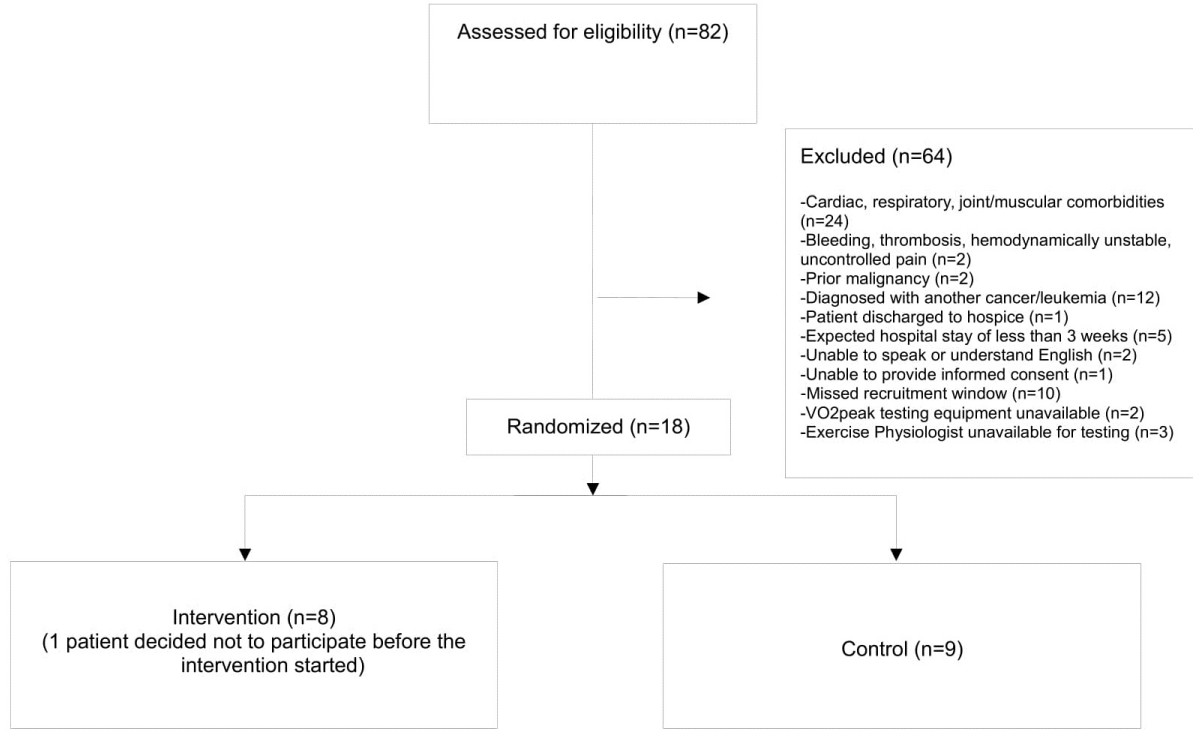
Figure 1: Screening for participation flow chart.
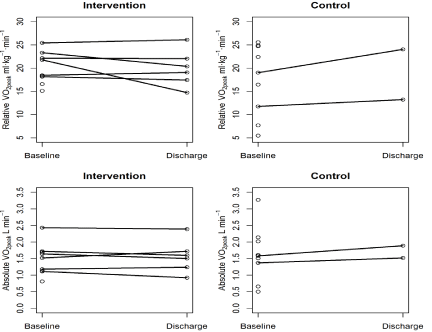
Figure 2: Baseline and Discharge VO2peak values expressed in relative (mL.
kg-1.min-1) and Absolute (L.min-1) at baseline and at discharge.
Eighteen patients were randomized to either the intervention (n=8) or control (n=9) group, one patient dropped out before the intervention began. After randomization, demographic and clinical characteristics, and patient-reported outcome measures were obtained. Patients were then scheduled to participate in physical function performance-based tests and a CPET before initiation of treatment and within 4 days of admission (Test 1) and postintervention testing was at the time of discharge (Test 2). At time of discharge, clinical characteristics as well as patient reported measures were collected again. Due to time and logistical constraints within the leukemia unit at the hospital, all functional and CPET testing were performed on a single day. Patients completed the 6-minute walk distance test (6MWT) and a Timed Up and Go test (TUG), then rested for at least 15 minutes before undergoing a CPET for both Test 1 and 2. Patient reported outcomes were collected weekly during hospitalization via paper surveys administered by the study coordinator. After baseline data were collected, all patients continued to undergo their standard of care for the treatment of leukemia with the only difference being that patients randomized to the intervention group began participation in the exercise training.
Measures
Cardiopulmonary exercise test (CPET) measure
CRF was evaluated using a CPET with indirect calorimetry for the assessment of peak oxygen uptake (VO2peak). The cardiopulmonary exercise test was performed at UNC Cancer Hospital hematology/ oncology unit, with a portable cycle ergometer (Monark 828E, Goteborg, Sweden). Expired gases were collected during the CPET using a portable mobile metabolic system (K4b2 Cosmed, Rome, Italy). The mobile metabolic system was calibrated prior to each test following manufactory specifications. The CPET was performed under the supervision of at least 3 trained exercise physiologists with medical support available if needed at any time during the test.
After receiving physician’s approval for participation, immediately before beginning the test, recent medical records were reviewed by the exercise physiologists and baseline vital sign measurements were obtained, including blood pressure, resting heart rate and oxygen saturation, as specified in pre-fitness testing guidelines from the American College of Sports Medicine [14]. The nursing staff was consulted regarding the current physical state of the patient prior to testing to ensure the patient had no pressing issue that could compromise participation in the CPET. If a patient had a fever or was sick the day of testing, the test was postponed but still kept within the 4 days admissions window. All patients were fitted for the cycle ergometer and for a mask to be used during the CPET for gas exchange analysis via indirect calorimetry. A resting metabolic measurement was obtained for approximately 5 minutes while patients sat quietly on the cycle ergometer prior to testing. Patients then begin pedaling at a cadence of 50 rpm for 3 minutes with no resistance as part of the test warm-up. The test started at a workload of 25 Watts and was increased in 5-20 watts/minute increments based on the initial response of the patient during the warm up period [6]. VO2peak was defined as the average of the 3 highest measurements of VO2 during the last stage where the patient reached volitional exhaustion and stopped the test, was no longer able to maintain a cadence of 50 rpm, and/or experienced any signs of symptoms that would precluded the patients to continue testing (chest pain, abnormal heart rate and blood pressure response to the exercise). Following achievement of VO2peak, subjects were asked to pedal with no resistance for approximately 2-3 minutes and were then assisted off the ergometer and seated while continuing to rest. Vital signs were re-evaluated and patients were assisted back to their rooms.
Physical function measures
Physical function assessments were performed to evaluate the efficacy of the exercise intervention in comparison to the control group. To assess functional capacity, patients participated in a 6-minute walk distance test (6MWT) using a 100-foot track on the hematology/oncology unit. Patients were instructed to wear clothing and shoes appropriate for walking exercise and were permitted to use their usual walking aids, including IV pole. They were instructed to complete as many laps as possible during the 6 minutes. The distance walked at the end of 6 minutes is termed the 6-minute walk test. After the 6MWT to assess functional capacity, patients were asked to perform a Timed Up and Go test (TUG) to assess mobility. To begin the test, the patient was seated in a standard armchair with their back against the chair. On command, patients would stand up, walk 3 meters at a comfortable pace, turn 180 degrees, walk back to the chair, and return fully to the initial seated position. A stopwatch as “time in seconds” measured results. The Karnofsky Performance Status tool (KPS) was used for the patient and provider to rate performance status [13,15]. The scoring ranges from 60-100 in increments of 10 with a higher score indicating better functioning.
Exercise training
Patients in the intervention arm participated in an individualized, mixed modality exercise program supervised by exercise physiologists for the duration of their inpatient hospitalization. Patients were approached 4 times a week, twice a day (AM and PM sessions) for aerobic (walking or stationary bike) and resistance training (use of different strengths of resistance bands). This progressive exercise model successfully used in one of our previous exercise trials in acute leukemia patients [10] consisted of aerobic training of 5-15 minutes and resistance training of 10-20 minutes. The aerobic exercise intensity progressed from approximately 50% to 70% of heart rate reserve by the end of the study, while all attempts were made to progress the resistance exercise intensity from lighter to heavier resistance bands using a 10 Rep Max (RM) training protocol. As patients were able to complete 3 sets of 10 repetitions maximum with a lighter band, a band providing greater resistance would used in subsequent workouts as an attempt to create a training load. Resistance exercises included lateral raises, frontal raises, chest press, low rows, biceps curls, triceps extension, leg extension and leg curl. In the morning session patients would undergo upper body exercises, and the afternoon session involved lower body exercises. Exercises were adapted based on the patient’s physical limitations. A cool down session included 5 minutes of stretching at the end of each session. Controls received the standard of care only and were monitored on their activity level during the hospitalization period using self-reported activity logs.
Prior to all training sessions, the exercise trainers would speak with the nursing staff regarding potential exercise contraindications for that day. Patients with platelet counts below <10×9/L, would not receive the intervention on that day. Patients experiencing low-grade fever (99-101°F) were allowed to exercise but at a lower training intensity and volume. Vital signs were collected before and after each session and reported to the nurse.
Data analysis
The pre-defined threshold for feasibility of the administration of a CPET during the study was set at 80% of all patients in both groups (intervention and control) being able to safely complete both CPETs at baseline and at discharge. Wilcoxon Rank Sum Tests, for continuous variables, and Fisher’s Exact tests, for categorical variables, were used to evaluate differences in baseline measures between the intervention and control group. Mean, standard deviations, and changes (Δ=Discharge–Baseline) in CRF, performance-based fitness function, and patient reported physical and mental health were calculated and presented for each group. Pearson correlation coefficients were used to explore relationships at baseline, discharge, and overall of CRF with physical function measures. Analyses were completed using SAS 9.4 statistical software.
Results
Sample characteristics
Patients included 17 adults (8 intervention and 9 control), ages 28-69 years. Mean age for intervention 52(13) and control 49(15) years. More than half (63%) were male, 21% minority, and most had a college or advanced degree. The mean number of comorbidities was 1.75 (range 0-5) for the intervention group and 3 (range 0-9) for control. The top comorbidities were arthritis (82%), hypertension (68%), anxiety (58%) and depression (58%). The majority had AML. BMI for the intervention group was 27.09(7.3) and 29.63(3.4) for the control group, indicating overweight status for majority of the patients. There were no statistically significant differences in sample characteristics between the intervention and control groups (Table 1 and 2).
Intervention
(N=8)
Control
(N= 9)
P-value
Age (years)
52 (13)
Range 34-67
49(15)
Range 28-69
0.85
Gender
Male
5 (62.5%)
7 (77.8)
0.62
Female
3 (37.5)
2 (22.2)
Race
Caucasian
6 (75%)
7 (77.8%)
0.26
African American
2 (25%)
2 (22.2)
Education
9th-11 grades
1 (12.5%)
0
High school
graduate/GED
1 (12.5%)
4 (44.4%)
Associate/Some
College
0
2 (22.2%)
College Degree
3 (37.5%)
1 (11.1%)
Advanced Degree
3 (37.5%)
2 (22.2%)
Income (household)
>20,000
2 (25%)
2 (22.2%)
0.46
20,001-40,000
1 (12.5)
4 (44.4%)
40,001-60,000
2 (25%)
1 (11.1%)
80,001-100,000
1 (12.5%)
2 (22.2%)
>100,000
2 (25%)
0
Marital Status
Single, never
married
1 (12.5%)
1(11.1%)
0.57
Married/Partnered
5 (62.5%)
7 (77.8%)
Divorced
2 (25%)
0
Widowed
0
1 (11.1%)
Clinical Characteristics
Type of acute leukemia
ALL
1 (14.3%)
1 (11.1%)
0.67
AML
7 (85.7%)
8 (88.9%)
Height (cm)
167.03 (12.3)
178.87 (14.1)
0.09
Weight (kg)
74.02 (20.5)
93.54 (19.5)
0.36
BMI
27.09 (3.4)
29.63 (7.3)
0.68
Table 1: Sample characteristics.
Intervention (n=8)
Control (n=9)
Variable
Baseline
Discharge
Change
Baseline
Discharge
Change
Rest
HR (BPM)
67(13)
79(18)
+12
80(13)
81(12)
+1
Systolic BP (mmHg)
128(22)
125(12)
-3
125(12)
136(22)
+11
Diastolic BP (mmHg)
69(15)
70(7)
+1
69(10)
71(10)
+2
SPO2 (%)
98(1.5)
98(1.6)
0
98(1.1)
98(1.0)
0
Resting Temperature (Co)
35.9(.58)
36.2(.47)
+0.3
36.1(.39)
36.6(.47)
+0.5
ANC (Ref:2-7.5 10×9/L)
1.52(1.08)
1.07(1.0)
-0.45
1.77(2.2)
1.36(1.5)
-0.41
Platelets (Ref:150-440 10×9/L)
51.67(30.2)
212.78(158.3)
+161.11
128(178.4)
109.6(106)
-18.4
Hb (Ref:13.5-17.5g/dL)
9.02(1.5)
8.53(3.2)
-0.49
9.4(1.07)
9.7(.73)
-0.30
Data Reported as Mean (SD); SPO2: Oxygen Saturation; ANC: Absolute Neutropenic Count; Hb: Hemoglobin; Ref: Reference Normal Values
Table 2: Baseline and discharge values for resting vital measurements.
Cardiorespiratory fitness (CRF)
Six out of 8 patients in the intervention group (75%) were able to complete both the baseline and discharge CPETs, while only 2 out of nine patients (22%) in the control group were able to complete both CPETs. No adverse event occurred in any of the baseline or discharge CPETs. The results of baseline, discharge, and change from baseline to discharge of the CPETs are presented in Table 3.
CPET
Intervention (n=6)
Control (n=2)
Variable
Baseline
Discharge
Change
Baseline
Discharge
Change
HRpeak (BPM)
152(28)
148(23)
-4
159(8)
167(23)
+8
VO2peak
mL.kg-1.min-1
21.5(2.8)
20(4.0)
-1.5
15.4(5.0)
18.6(8.0)
+3.2
VO2peak
L.min-1
1.60(0.40)
1.56(0.49)
-0.4
1.48(0.14)
1.71(0.26)
+0.23
Workload (Wattpeak)
96(63)
90(60)
-6
99(46)
115(42)
+42
Borg
RPE (6-12)
16(3)
16(3)
0
14(1)
16(2)
+2
Data Reported as Mean (SD).
Table 3: Cardiopulmonary exercise (CPET) test parameters at baseline and at hospital discharge.
Physical function measures
The results of baseline, discharge, and change from baseline to discharge for all physical function tests are presented in Table 4. One intervention patient did not complete the discharge TUG, two intervention patients did not complete the discharge 6MWT. Two control patients did not complete either the discharge TUG or 6MWT.
Physical Function
Functional Tests
Intervention (N=8)
Control (N=9)
Baseline
Discharge
Change
Baseline
Discharge
Change
6MWT (Meters)
491.3(100.8)
524.2(108.6)
+32.93
401(67.7)
424.5(90.1)
+23.5
Timed Up and Go (Seconds)
8.4(2.1)
7(3.4)
-1.39
9.3(2.7)
9.2(2.7)
-0.11
Karnofsky Performance Status (60-100)
90(12.0)
91.3(6.4)
1.25
87.8(12.2)
83.3(14.1)
4.44
Table 4: Physical function assessments.
Figure 3 below illustrates the results of the physical function measures administered to patients in the intervention and control groups at baseline and at discharge from the hospital.
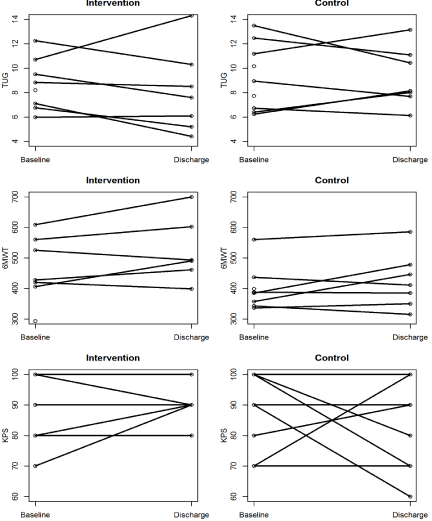
Figure 3: Physical Function Measures.
A) TUG: Timed Up and Go test (Seconds); B) 6MWT: 6 Minute Walk Test
(Distance in meters); C) KPS: The Karnofsky Performance Status (Score)
Correlation between values obtained at baseline and discharge for all patients CRF and Physical measures
A significant inverse correlation was observed between CRF and TUG (r=-0.67, p=0.0003), indicating that the higher the VO2peak, the faster the time (better performance) on the TUG test. A significant correlation was also observed between CRF and the 6MWT (r=0.61, p=0.001), meaning that the higher the VO2peak, the greater the distance walked. No significant correlation was observed between CRF and the Karnofsky Performance Status Questionnaire.
Figure 4 and 5 depicts the correlation between CRF and TUG, CRF and 6MWT.
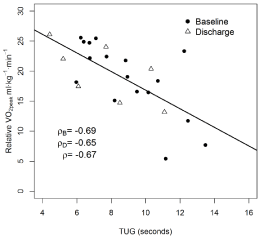
Figure 4: Correlation between CRF and TUG at baseline (circle) and after
discharge (triangle).
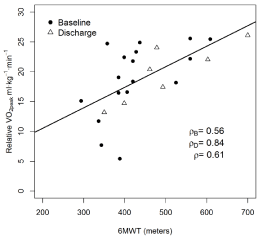
Figure 5: Correlation between CRF and 6MWT.
Discussion
Cardiorespiratory Fitness expressed as maximal oxygen uptake is a fundamental measurement of cardiovascular health/function both in healthy as well as in diseased populations. Fairly recently, CRF has been shown to be a strong independent predictor of survival in different cancers [6,8] and could potentially be used as another parameter to improve risk stratification and prognostication in oncology patients. However, the administration of a CPET for the assessment of CRF expressed as maximum oxygen uptake in the clinical setting and for certain clinical populations can present challenges that may make this important measurement difficult to attain. From the logistics of space, equipment, specialized personnel, and scheduling complications of physicians and nurses, the process of assessing CRF in the clinical setting becomes even more difficult and complex. Due to the nature of the disease process and the need to initiate chemotherapy treatment immediately post-diagnosis, there is a need to investigate the feasibility and safety of conducting a maximal CPET in this specific cancer population. Therefore, we examined the feasibility of conducting a maximum CPET in newly diagnosed acute leukemia patients enrolled in a randomized clinical trial examining the effects of an exercise program during induction chemotherapy.
In our study, the feasibility of conducting a CPET in newly diagnosed acute leukemia patients initiating induction chemotherapy should be interpreted in a way that not only clinicians can benefit from the information but also from a research perspective where CPETs are often used as a way to quantify objectively the effects of exercise on CRF after chronic training. From a perspective of using a CPET as a prognostic parameter to predict potential treatment complications and survival, our study showed that it is not only feasible but also safe to conduct a CPET in the hospital in newly diagnosed acute leukemia patients. All patients enrolled in the study (N=17) were able to undergo a CPET prior to the beginning of induction chemotherapy with no adverse events observed. However, after the conclusion of the exercise training at time of discharge from the hospital, only 8 patients out of the 17 (N=6 intervention and N=2 control groups) were able to complete both baseline and discharge CPETs. This corresponds to all patients only participating in 47% of all planned baseline and discharge CPETs (16 out of 34 planned tests). The 2 patients in the control group who were able to participate in the CPET at their discharge from the hospital engaged in regular physical activity and therefore cannot be seen as true control subjects in this study. These 2 patients reported to the research team that they were walking most of the days of the week, sometimes 2 times per day.
Based on the feasibility criterion adopted in this study, it does not seem feasible to perform CPETs at baseline and at discharge in newly diagnosed acute leukemia patients during induction chemotherapy. However, it is very important to note, that 75% of patients enrolled in the exercise group of the randomized controlled exercise trial were able to complete baseline and discharge CPETs. This confirms the results of previous studies [9-12] where exercise training during induction chemotherapy helps reduce decline of physical conditioning allowing for patients to maintain a great level of overall functional capacity when compared to patients who do not exercise while in treatment. The reason the 2 patients in the intervention group did not participate in the discharge CPETs was because they were discharged from the hospital earlier than expected and our team was unable to schedule the discharge CPET; however, they were able to complete their 6MWT. For the control group, most patients declined to participate in the discharge CPET due to fatigue, the desire to leave the hospital as soon as possible, and also no interest in undergoing another CPET.
The 6MWT is a very simple and easy test to administer for the assessment of functional capacity and has shown to be a good predictor of mortality in different populations [6-8]. It has also been shown to be clinically feasible to patients who are extremely deconditioned such as those with chronic obstructive pulmonary disease (COPD), and patients with cardiac disease [15-17]. In the current study, all but 2 patients in the control group (who did not complete the discharge tests) completed the baseline and discharge 6MWT (94% of planned tests). The 2 control patients did not complete the discharge 6MWT because they did not want to wait to undergo discharge testing before going home.
Exploratory analyses evaluating the relationships between the results of all the CPET and physical function tests conducted during the study showed that the 6MWT and TUG physical function tests correlated well with the CPET in newly diagnosed leukemia patients, while the KPS did not. Since the adherence to the 6MWT and TUG was greater compared to the CPET, we recommend that 6MWT and TUG be used for future studies evaluating the effects of an exercise on physical function as they appear to be the best options. However, it would be also important to examine the value of a CPET administered prior to treatment of even an exercise intervention as a potential predictor of prognosis that could be added to other current risk factors of survival in this cancer population. For studies looking into examining the effects of exercise on CRF, it is important to note that in this current study, VO2peak was significantly correlated with the TUG and 6MWT, and we therefore recommend the use of the TUG and 6MWT instead of the CPET in this particular population when the goal is to evaluate the effects of an exercise intervention in clinical control trials on overall physical function.
In oncology practice, physical functioning is usually evaluated using subjective systems such as the Karnofsky Performance Status (KPS). The KPS is also used to classify patients into prognostic risk categories, which helps to inform major decisions on the use of different treatment options. In our study, the only measurement not significantly correlated to the results of the CPETs was the KPS. Due to the subjective nature of the KPS, the measurement may lack sensitivity to provide a precise evaluation of physical function of newly diagnosed leukemia patients. These limitations with the KPS have been postulated in a previous study with patients recurrent of glioma (19) and appear to also apply to leukemia patients. Therefore, it is recommended that an objective measurement such as VO2peak, the 6MWT or TUG be used in combination with the KPS with the goal to provide a more precise physical function characterization in leukemia patients. Due to the fact that the 6MWT and TUG tests are easy to administer in clinic and to date very few studies have looked into the prognostic value of using the 6MWT and TUG in oncology patients [14,20,21], the prognostic value of these tests should be further explored.
In conclusion, there were no adverse events during any CPET conducted during the study confirming that it is safe to administer a CPET in newly diagnosed leukemia patients during induction therapy. Based on the criteria used to determine feasibility of conducting a CPET at baseline and discharge, the results of this study demonstrate that it is not feasible. It seems that the patients in the standard care with no exercise (control group) experienced significant functional that precluded their ability or willingness to undergo the CPET prior to discharge from the hospital. The results of this study should be interpreted cautiously due to the relatively small sample size, but provide preliminary evidence that the use of CPETs to assess the effects of an exercise program in acute leukemia patients during induction therapy on CRF while safe, may not be feasible. For future studies examining the effects of exercise on CRF in newly diagnosed leukemia patients, the use of a CPET for the assessment of VO2peak along with the 6MWT and/or the TUG test in a larger sample is recommended so the results of this preliminary study can be confirmed of refuted.
Acknowledgments of Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA116339, the UNC University Cancer Research Fund UL1RR025747, and NCI 5K12CA120780-07.
References
- Society AC. Cancer Facts & Figures 2016. 2016.
- Battaglini CL. Physical activity and hematological cancer survivorship. Physical Activity and Cancer: Springer. 2010; 275-304.
- Morris JN, Heady J, Raffle P, Roberts C, Parks J. Coronary heart-disease and physical activity of work. The Lancet. 1953; 262: 1111-1120.
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002; 346: 793-801.
- Ekelund LG, Haskell WL, Johnson JL, Whaley FS, Criqui MH, Sheps DS. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men. N Eng J Med. 1988; 319: 1379-1384.
- Jones LW, Watson D, Herndon JE, Eves ND, Haithcock BE, Loewen G, et al. Peak oxygen consumption and long-term all cause mortality in non-small cell lung cancer. Cancer. 2010; 116: 4825-4832.
- Wood WA, Deal A, Reeve B, Abernethy A, Basch E, Mitchell S, et al. Cardiopulmonary fitness in patients undergoing hematopoietic SCT: a pilot study. Bone Marrow Transplant. 2013; 48: 1342-1349.
- Jones LWaBCL. Exercise Testing in Cancer Patients; ACSM’s Guide to Exercise and Cancer Survivorship. Lippincott Williams & Wilkins; 2012.
- Chang PH, Lai YH, Shun SC, Lin LY, Chen ML, Yang Y, et al. Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: a randomized controlled trial. J Pain Symptom Manage. 2008; 35: 524-534.
- Battaglini CL, Hackney AC, Garcia R, Groff D, Evans E, Shea T. The effects of an exercise program in leukemia patients. Integr Cancer Ther. 2009; 8: 130-138.
- Klepin HD, Danhauer SC, Tooze JA, Stott K, Daley K, Vishnevsky T, et al. Exercise for older adult inpatients with acute myelogenous leukemia: a pilot study. J Geriatr Oncol. 2011; 2: 11-17.
- Alibhai SM, O’Neill S, Fisher-Schlombs K, Breunis H, Brandwein JM, Timilshina N, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012; 36: 1255-1261.
- Jarden M, Møller T, Kjeldsen L, Birgens H, Christensen JF, Christensen KB, et al. Patient Activation through Counseling and Exercise–Acute Leukemia (PACE-AL)-a randomized controlled trial. BMC Cancer. 2013; 13: 446.
- Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription. Curr Sports Med Rep. 2013; 12: 215-217.
- Karnofsky DA. The clinical evaluation of chemotherapeutic agents in cancer. Evaluation of chemotherapeutic agents. 1949.
- Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF, et al. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med. 2006; 100: 1734-1741.
- Flaherty KR, Andrei AC, Murray S, Fraley C, Colby TV, Travis WD, et al. Idiopathic pulmonary fibrosis: prognostic value of changes in physiology and six-minute walk test. Am J Respir Crit Care Med. 2006; 174: 803-809.
- Cote CG, Pinto-Plata V, Kasprzyk K, Dordelly LJ, Celli BR. The 6-min walk distance, peak oxygen uptake, and mortality in COPD. Chest. 2007; 132: 1778-1785.
- Jones LW, Cohen RR, Mabe SK, West MJ, Desjardins A, Vredenburgh JJ, et al. Assessment of physical functioning in recurrent glioma: preliminary comparison of performance status to functional capacity testing. J Neurooncol. 2009; 94: 79-85.
- Ruden E, Reardon DA, Coan AD, Herndon JE 2nd, Hornsby WE, West M, et al. Exercise behavior, functional capacity, and survival in adults with malignant recurrent glioma. J Clin Oncol. 2011.
- Kasymjanova G, Correa JA, Kreisman H, Dajczman E, Pepe C, Dobson S, et al. Prognostic value of the six-minute walk in advanced non-small cell lung cancer. J Thorac Oncol. 2009; 4: 602-607.