
Special Article - Leukemia
Ann Hematol Oncol. 2018; 5(2): 1192.
Acute Leukemia Patients: A CLABSI Risk Special Population
Martinez JM¹*, Santo AE², Godinho A³, Azevedo A4, Felix A5, Chacim S6, Ramada D7, Mariz JM8 and Medeiros R9
¹Registered Nurse, Clinical Nurse Specialist, Hematology- Oncology Department, Instituto Português de Oncologia, Porto
²Hematology-Oncology Department, Molecular Oncology Group-CI, Portuguese Institute of Oncology, Porto
³Registered Nurse, Hematology-Oncology Department, Portuguese Institute of Oncology, Porto
4Registered Nurse, Clinical Nurse Specialist, Hematology- Oncology Department, Portuguese Institute of Oncology, Porto
5Registered Nurse, Hematology-Oncology Department, Portuguese Institute of Oncology, Porto
6Hematology-Oncology Department, Portuguese Institute of Oncology, Porto
7Registered Nurse, Clinical Nurse Specialist, Day Hospital Department of Portuguese Institute of Oncology, Porto
8Hematology-Oncology Department Director, Portuguese Institute of Oncology, Porto
9Molecular Oncology Group-CI, Portuguese Institute of Oncology, Porto
*Corresponding author: Martinez JM, Hematology- Oncology Department, Instituto Português de Oncologia- Porto, R. Dr. Ant Bernardino de Almeida, 4200-072 Porto, Portugal
Received: January 11, 2018; Accepted: February 12, 2018; Published: February 28, 2018
Abstract
Background: Patients with acute leukemia (AL) have a higher risk of neutropenia. Central venous catheters (CVC) are indispensable devices during chemotherapy treatments and aplasia support.
Methods: This is a single-center, retrospective cohort study, and reporting 154 hospital admission episodes, either for chemotherapy treatment or aplasia support, regarding twenty-eight AL patients using a Hickman CVC for more than 72h, from January 2013 to December 2015.
Results: Overall 3032 CVC manipulations considering a median of 1 CVC manipulations by catheter/day (range, 5 to 0) among 2130 hospital admission days (2007 catheter days) were reported. CLABSI was always identified in neutropenia admissions (1212 neutropenia-days) within cases presenting a median number of CVC manipulations superior to 15. The number of CVC manipulation increases along with cumulative neutropenia days [r=0.752, p=0.000 with an R² = 0.605]. However no relation was found with the cumulative non-neutropenia days. Taking neutropenia condition into account, CLABSI risk is increased considering cumulative CVC manipulations [CLABSI group, mean±SD, 27.89±3.199; non-CLABSI group, mean±SD, 20.82±1.189; p=0.046]. No CLABSI was identified after the first positivity blood cultures result and no CLABSI was reported by ANC>500 cells.
Conclusion: We conclude that in neutropenic patiens, undergoing induction therapy or in aplasia support, CLABSI risk increases along with cumulative neutropenia days prior CLABSI and CVC manipulations being the AL patients could be considered a CLABSI risk special population.
Keywords: CLABSI; CRBSI; Acute leukemia; Hickman catheter; Neutropenia
Introduction
Infectious diseases are important causes of both morbidity and mortality in hematology oncology patients. Patients with acute leukemia (AL) have a higher risk of neutropenia due to high-dose chemotherapy treatments and to malignancy itself [1]. Multiple chemotherapy cycles, antibiotic resistant bacteria, and high transfusion rates are known predisposing factors that increase the incidence and prevalence of bloodstream infections (BSI) [1].
There are four major catheter types based on their designs: Nontunnelled CVCs, Tunnelled CVCs (i.e., Hickman or Broviac catheters), Implantable ports and peripherally inserted central catheter (PICC) [2]. The placement and type of CVC depends on the preferences of the patient, the healthcare provider and the IV therapy duration [3]. The Healthcare and Technology Synergy (HAST) framework considers patient, product and practice as the central elements of effective clinical research associated with central venous catheters (CVC) [4]. The real value of CVC management still remains unclear due to the low description and few management details reports [5-6].
Catheter-related occlusion due to mechanical obstructions and catheter-related infection are the most important complications in the management of the central venous devices [7-9]. In AL inpatients the risk of these complications is high due to myelossupresion, especially neutropenia and thrombocytopenia [10]. The most common modifiable risk factors known to increase overall catheter-related bloodstream infection (CRBSI) are CVC-life, parenteral nutrition, multi-lumen CVC, high workload or CVC-associated thrombosis, being the imunocompromised status the highlighted non-modifiable risk factor in hematology oncology patients [1-11]. The principal way for healthcare professionals to reduce and control the pathogenesis of infections in central line devices are the insertion and maintenance procedures [4,6-8]. Several studies report behavioral changes, education of healthcare professionals, insufficiently trained nurses, a low nurse-to-patient ratio and protected environments directly related to infection control strategies [7,12].
This clinical research studies neutropenia and CVCmanipulations associated with central-line associated bloodstream infection (CLABSI) using evidence-based science.
Material and Methods
Selection and description of participants
A single-centre, retrospective cohort study was performed, including all consecutive AL patients using a Hickman CVC for more than 72h, undergoing chemotherapy treatment (CT)or aplasia support from January 2013 to December 2015 at the Haematology Department of the Portuguese Institute of Oncology (Porto).
Patients older than 18 years old with newly diagnosed or relapsed acute leukemia admitted for CT or aplasia support and with a CVC inserted during the study period were included. Patients in supportive care, who had previous hematopoietic stem cells transplantation, with clinical septicemia at the moment of the CVC introduction, with insertion procedure complications or with acute promyelocytic leukemia diagnosis were excluded. One hundred and twenty three hematology-oncology patients placed a long-term catheter in the study period, AL (n=32). After inclusion and exclusion criteria (septicemia n=3, insertion procedure complication n=1), twenty eight AL patients with a Hickman catheter were included.
Data collection
Data concerning each patient’s background was collected from the medical records. The daily data assessment ended when the CVC was removed for sepsis or end of treatment. When the final eligible patient was admitted to the study a minimum of one-month follow up was considered. The baseline demographic data was collected on the day of CVC placement and assessment was encompassed in every hospital admission.
Neutropenia and central-line infections definitions
Neutropenia [13] was considered when ANC (Absolute Neutrophil Count) =500 cells, or when no differential count was available and WBC (White Blood Count) =1600 cells was reported (previous statistical correlation analysis). A total of continue neutropenia-days was considered duration of neutropenia. Overall neutropenia-days related to catheter-days were considered Neutropenia Ratio (NR). Neutropenia days-prior CLABSI were considered the number of neutropenia-days since the first neutropenia-day to CLABSI reported.
CLABSI and CRBSI rates were calculated considering BCs yielding an organism (positive culture in peripheral vein and at least one CVC-line) per 1000 CVC-days. CLABSI was considered in patients with a central line in place within 48-hour period and bloodstream infection that is not related to an infection at another site [12]. Differential Time Positivity was reported (samples from CVC lines become positive 120 minutes or more before peripheral vein samples), CRBSI was considered [12]. The ratio between the mucosal injury barrier microorganism (MBIm) [14] and total of microorganism recovered was considered MBIm ratio.
CVC manipulations and catheter-related occlusions definitions
Manipulation was considered in every approach to CVC with at least one open line. One manipulation of the CVC was considered every time the CVC line was opened to change the administration sets, collect blood samples or blood cultures (BC). When transfusion support was performed two manipulations were considered.
Occlusion was considered when the capacity to blood withdrawal was compromised and the ability to flush fluids is lost. Partial occlusion (inability to aspirate blood but ability to infuse through the catheter) and complete occlusion (inability to aspirate blood and infuse through the catheter) were reported. Catheter-related occlusion was calculated considering the occlusion events per 1000 CVC-days [2,9]
Technical department information
The department consisted of 20 beds distributed among eight double and four single rooms, all equipped with positive pressure ventilation and HEPPA filters. The insertion of CVCs is performed by medical staff in an operating room [7] located in the department, and daily management of CVCs is performed by nursing staff. During the study period, no other relevant departmental changes were implemented, including CVC insertion, CVC management procedures, indication for BC, and BC assessment.
Blood cultures collection and empirical antibiotic use policy
BC collected by control indication and non-department and hospital acquired infections were not included in the study [12]. For every episode with an indication for BCs, samples were collected first from a peripheral vein followed by the CVC line with no more than five minutes between samples to reduce DTP results bias [12-15]. BC collection was performed by one nurse. BC samples were collected with a minimum of 5mlof blood, when possible, in BACTED PLUS Aerobic/F® vials and analyzed by the microbiology department. In an attempt to reduce false positive BC results due to positive needleless connector and negative hub contamination, needleless connectors were removed before collecting BC samples. Large spectrum antibiotherapy was started as per the 2009 Infectious Diseases Society of America Guidelines for Intravascular Catheter-related Infection, recommended to treat gram-negative bacterial infections in patients undergoing neutropenia or septicemia special conditions [16].
Device management
The management of CVCs followed the CDC (2011) guideline recommendations [13]. Hickman catheters (Vygon®) without any antimicrobials were inserted in the subclavian vein. All catheters were double lumen (CH/F 7, lumen no.1=0.6, lumen no.2=1.0). No antibiotic prophylaxis was performed. Specific technical information of CVC management included the use of: chlorhexidine 2% in alcohol 70% solution for needleless connector disinfection, split septum needleless connector (Bionecteur, Vygon®) and sodium heparin 20 IU/ml (Fibrilin®) to CVC-lock.
Data analysis
Data analysis was conducted using IBM SPSS Statistics for Windows (SPSS Inc., Version 24.0) licensed by ICBAS-UP (Instituto de Ciências Biomédicas Abel Salazar-Universidade do Porto; Master Degree Oncology Program. A continuous variable was reported by median or mean (when appropriate) and range. Categorical variables were reported as frequency and percentages. Any association between two continuous quantitative variables was analyzed by Pearson´s test. Normality tests reported a sample without normal distribution, considering that hypothesis tests were analyzed by non-parametric test. A p value of =0.05 was determined to be significant.
Protection of personal data
The study was approved by the Ethics Committee (CES IPO: 137/2016) of the Portuguese Institute of Oncology (Porto) on 16 June, 2016. All data was treated in compliance with the Portuguese Law n° 67/98 of 26 October concerning the protection of personal data.
Results
A total of 28 patients diagnosed with AL among 154 hospital admission episodes related by CLABSI/non-CLABSI [13(8.4%)/141(91.6%)] were reported (Table 1). Among this sample, median age of 49 years [range, 68 to 23] summarized in 21(75%) female and 7(25%) male patients were identified. No CLABSI risk were found considering diagnose [RR 0.976, 95% CI, 0.887-1.074], relapse [RR 2.267, 95% CI, 0.688-7.472], age [=50/>50 years, reference group] [RR 0.922, CI 95%, 0.325-2.618] and gender [RR 2.000, 95% CI, 0.663-6.034]. Reasons for hospital admission were induction [32(20.8%)], aplasia support [48(31.2%)], CT [70(45.4%)], and CT + aplasia [4(2.6%)].
Characteristic
AL
AML
ALL
p
Patients, n(%)
28
17(67.7)
11(39.3)
0.345
Admissions, n(%)
154
97(62.9)
57(27.1)
0.002
Relapse Yes
No
18
136
18(11.7)
79(51.3)
0(0)
57(37)
0.000
0.072
Age, years, median (range)
49(68-23)
49 (68-25)
53(66-23)
0.750
Gender Male, n(%)
Female, n(%)
21(75)
7(25)
10(6.5)
87(56.5)
18(11.7)
39(25.3)
0.186
0.000
Admission days(ID), n[median(range)]
2130
1470[12(42-5)]
660[7(38-4)]
0.001
CVC days, n[median(range)]
2007
1394[12(38-5)]
613[7(35-4)]
0.000
ANC = 500 cells days n[median(range)]
1212
943[15 (38-2)]
269[10(33-1)]
0.002
Neutropenia Ratio
0.60
0.68
0.44
NA
Number of CVC/patient, median(range)
42
1(4-1)
1(2-1)
0.200
ALL: Acute Lymphocytic Leukemia; AML: Acute Myeloid Leukemia; NA: Not Applicable
Table 1: Baseline Characteristics.
Catheter baseline characteristics and infection risk
In total, 42 Hickman catheters (median 1; range 4 to 1) were inserted, concerning 2130 hospital admission days (ID) including 2007 catheter days. On admission, the CVC was placed in =7 days in 90.5% events (median 1, range 17 to 0). No CLABSI was observed in CVC placed after >7 days of admission. Subclavian right side was considered in CVC placement among 36(85.7%) events. No statistical significance related to CLABSI was found regarding CVC laterality, p=0.463. The CVC was removed at the end of treatment in 32(76.2%) cases. Other reasons for removing CVC lines were septicemia, observed in 5(11.9%) cases, followed by insertion site infection in 4(9.5%) cases. In only 1(2.4%) event, the CVC was removed after patient death. Twelve CVCs had more than 100 days-life (median 67, range 188 to 8). No admission in intensive care department related to CLABSI was reported.
Catheter-related occlusions
A total number of 16 occlusion days (median 1, range 5 to 1) among 10 occlusion events were observed. Partial occlusion was identified 8(80%) times (median 1, range 2 to 1), being complete occlusion reported 2(20%) times (median 3.5, range 5 to 2). Considering platelets count at the occlusion day, no significant differences were found between partial (median 26.5, range 306 to 7) and complete (median 155.5, range 168 to 143) occlusions (p=0.400). Whereas placement CVC day, a median of 67.5 (range 172 to 17) days to the occlusion were reported. Considering the hospital admission days related occlusion, earlier occlusion events (=72h) were observed (median 3, range 27 to 0) [p=0.010], always reported in induction with a 15 hospital days’ time superior [RR 3.000, CI 95%, 0.914 to 3.000]. No occlusion after transfusion support or 72h after and before CLABSI was observed. No catheter-related thromboses reported. Overall 4.98 catheter-related occlusion rates per 1000 catheter-days, including partial 3.98 and complete 0.99, were reported.
Neutropenia, CVC manipulations and CLABSI risk
An overall of 1393 blood samples, 595 transfusions, 145 BCs, and 304 CVC-line isolated substitutions were observed. Overall 1212 neutropenia-days were reported. Considering the duration of neutropenia, induction reported superior median of neutropeniadays (median 19, range 38 to 1) than aplasia support (median 12, range 23 to 3) [p=0.000]. When neutropenia-days prior CLABSI is considered, no statistical significance between induction (median 6.5, range 13 to 2) and aplasia support (median 4, range 9 to 1) was observed, [p=0.285].
A total number of 3032 CVC manipulations were reported (median 15, range 95 to 3) considering a median of 1 CVC manipulations by day (range, 5 to 0). CVC-lines were used with perfusion iv (at least one day) in 142 hospital admissions, being only used to blood samples and transfusion support in 12 aplasia support admissions without CLABSI reported.
CLABSI was always reported in neutropenia admissions within cases presenting a median number of CVC manipulations superior to 15. The number of CVC manipulation increases concerning cumulative neutropenia days [r=0.752, p=0.000 with a R²=0.605]. However no relation was found with the cumulative non-neutropenia days [r=0.051, p=0.564 with an R²=0.004]. Taking neutropenia condition into account, CLABSI risk is increased considering CVC manipulations [CLABSI group, mean±SD, 27.89±3.199; non-CLABSI group, mean±SD, 20.82±1.189; p=0.046] (Figure 1).
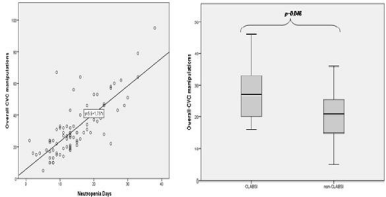
Figure 1: CVC manipulations correlated to neutropenia days (left); CVC manipulations and CLABSI risk in neutropenia (right).
Considering the induction phase as reference, manipulation ratio (median of manipulations related by phase) (induction=1, aplasia support=0.45, CT=0.12) was reported. No statistical significance was found related to the number of CVC manipulations in neutropenia days prior CLABSI considering induction (median 10, range 12 to 2) and aplasia support (median 7, range 15 to 2) p=0.762. No significant CLABSI risk association between induction and aplasia support was found (RR 0.736, 95% CI, 0.311-1.745) (Figure 2).
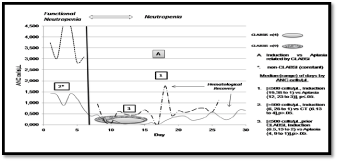
Figure 2: CLABSI and ANC related by hospital admission.
Blood cultures and microbiological recovery
BCs were collected in 48.7% (median 0, range 6 to 0) of all hospital admissions, being positive in 21(10.4%) cases. No positive BCs were identified after the first positivity result; after a first negative result, 2(9.5%) positive BCs were reported. Considering BCs related by ANC, no positive BCs were reported by ANC >500 cells in AML patients, and only 1(4.7%) positive BC was reported by ANC >500 cells in ALL patients. Considering CVC-lines microorganism recovery (peripheral vein always positive in CLABSI reports), 1.0 CVC-line was colonized in 12(92.3%) of cases when CLABSI reported. In the particular case of colonization recovery (peripheral vein always negative in colonization reports), 0.6 CVC-line always was colonized in all cases 5(100%). The only gram-negative bacteria reported by ALL patients were Klebsiella Pneumoniae, being the rest of gram-negative bacteria associated to AML patients. E.coli 4 (30.7%) was the most representative microorganism identified in CLABSI events, being the Klebsiella Pneumoniae 3(60%) the most representative microorganism linked to CVC colonization events. The study suggests an overall 0.80 MBIm ratio associated to colonization events. No fungus was identified. Overall, CRBSI and CLABSI rates per 1000 catheter-days were reported as 0.49 [AML 0 and ALL 1.63) and 6.47 [AML 6.45 and ALL 6.52], considering overall 0.63 MBIm ratio associated [AML 0.62 and ALL 0.69].
Discussion
AL patients undergoing chemotherapy treatment are a special oncology-hematology population. The admissions are naturally linked to multiple CVC manipulations. Infection control CVC measures such as blood samples drawn at the moment of CVC line change, CVC line maintenance every 72h (changing needleless connectors), optimal choice of needleless connectors used (positive pressure mechanical valves are associated to high infection rates) [12,17,18], turbulent flush and positive-pressure locking techniques could reduce unnecessary CVC manipulations and CLABSI rates [19]. Large osmolarity spectrum drugs, several infusion and perfusion volumes and lower thromboses rates suggested Hickman catheters to be the main vascular access devices in AL patients undergoing high dose CT since 80´s decade [2]. Ming Y. Ling, et al. 2013, developed at Mayo Clinic Rochester a clinical research comparing the efficacy of 84 Hickman Catheters versus 64 PICCs in the treatment of AML patients. The study reported no significant differences in catheterrelated thrombosis, central-line associated bloodstream infections (CLABSI) and CRBSI rates. However, catheter-related occlusion was significant higher in PICCs (20.43 versus 1.25 per 1000 CVC-days, p=.0001) [2].
Neutropenia and CVC manipulations related to CLABSI risk
Neutropenia is considered a major CLABSI risk factor [2,10,13,17]. This study suggested that aplasia (neutropenia, anemia, and thrombocytopenia) increases the number of CVC manipulations, mostly due to several blood samples, transfusion support and BCs collection. This study reported a manipulation ratio higher in induction than in aplasia support and CT. However, similar CLABSI rates were reported between induction and aplasia, being a CLABSI considered a rare event in CT. This could be explained considering that cumulative neutropenia days increases the number of CVC manipulations. This fact associated to neutropenia increases CLABSI risk. In consequence, this study suggests that neutropenia and CVC manipulations association are major CLABSI risk factors. Therefore, when appropriated CVC management, isolated or cumulative CVC manipulations in non-neutropenia could be considered minor CLABSI risk factors. Considering neutropenia-days prior to CLABSI and biofilm formation risk period (48/72-hour period) [18,19], risk phase is higher between days 9 to 15 in induction and days 1 to 7 in aplasia support (Figure 3).
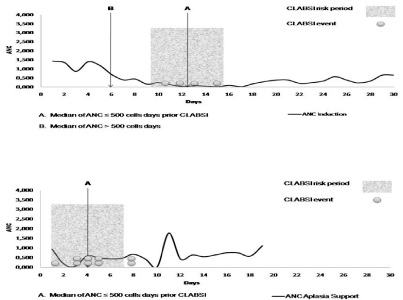
Figure 3: CLABSI risk period related to neutropenia hospital admissions: Induction and aplasia support.
CVC management: solution-lock and needleless connectors
Catheter-related occlusion and infection should not be dissociated [9]. An adequate clean sweep of the catheter-lumen using sodium chloride 0.9% through push-pause technique seems to be essential to secure CVC pattency and reduce biofilm formation risk [12,19-20]. Theoretical rationale studies support that using heparin to CVC-lock can reduce catheter-related thrombosis and fibrin deposition (formation film). Bradford, et al. 2016, in their systematic review “Heparin versus 0.9% sodium chloride intermittent flushing for the prevention of occlusion in long term central venous catheters in infants and children” published in “The International Journal of Nursing Studies”, report that most of institutions recommend the use of heparin when CVC is not in use. The study reports that clinical research is associated with a quality study ranged from low to very low evidence. Indeed, different protocols with several concentrations and frequencies of heparin were related. Finally, this study concludes that more well-designed researches are required [21].
Alberto Dal Molin, et al. 2015, suggested that normal saline flushing in totally implanted venous access devices is not inferior to heparin flushing (with a study power lower than 56%). In the case of occlusion types, study results revealed a partial occlusion more frequent than complete, being only one complete occlusion observed in the saline group. The study did not include AL patients and did not consider neutropenia condition. The authors present the clinical study of Cesaro, et al. 2009, where 203 pediatric patients were randomized in a trial that revealed an increased-rate of complications in patients using Broviac-Hickman catheters flushing with normal saline solution [22]. Healthcare professionals avoid heparin due to the heparin-induced thrombocytopenia [12], however in the particular case of AL patients, thrombocytopenia is considered a frequent condition. Abdelkefi, et al. 2007, studied 246 patients with non-tunneled central venous catheters comparing the use of continuous infusions of heparin and low-dose unfractionated heparin to prevent CRBSI. The study did not reported heparin-induce thrombocytopenia and severe bleeding complications between groups (p=1.00) [23].
This study reports the use of heparin to CVC-lock after catheterlumen clean sweep. Considering heparin short-life, low heparin concentration (20-30 UI) and non-bleeding reports, the use of heparin to CVC-lock could be considered of dismal risk. In this study we indicate that heparin could reduce cumulative fibrin deposition (possible biofilm and thrombi formation source) [12,18,19] in the first hours after CVC manipulation based on the CRBSI and catheter-related occlusion rates reported. Mauro Pittiruti, et al., 2016, developed a consensus for the choice and the clinical use of the most appropriate lock solution for central venous catheters (excluding dialysis catheters). The study concluded that the value of the heparinization for non-dialysis catheters should be reconsidered [24].
In the last decade the MVC-PP replaced SSNC to reduce the use of heparin and catheter-related occlusions [18]. The design of an MVC-PP versus SSNC showed an important structural difference between them: the MVC-PP allows the fluids to enter and return inside the connector through the internal valve; on the other side the SSNC allows the fluids to enter and return inside the connector without resistance. Flushing CVC above 0.1 ml is enough to create positive pressure in MVC-PP and if just a little more product is infused it is expelled. Even using heparin CVC-locks, biofilm formation risk related to cumulative fibrin deposition through the internal valve seems to be on the basis of superior infection rates associated with MVC-PP [25]. The Centers for Disease Control and Prevention, 2011, Guidelines for the prevention of intravascular catheter-related infections, reported the increased risk of infection with the mechanical valves devices used (category II). 12 One year later, the Joint Commission International Monography, Preventing central line-associated bloodstream infections: a global challenge, a global perspective, reported 4 studies supporting this data [2].
Using SSNC, CVC-line clamp before connector syringe withdrawal could be considered a positive-pressure technique (Figure 4). When the syringe is removed, empty space generated inside the connector is created. It does not allow the blood reflux into the catheter tip, and consequently the probability of occlusion by small thrombi formation is reduced. In the particular case of partial occlusions reported, the study reported the resolution of all events in less of 48-hours. In consequence, this study suggested that the use of heparin to CVC-lock could help to reduce and resolve partial occlusions.
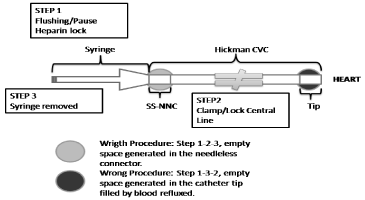
Figure 4: CVC-line clamp positive-pressure technique.
Scope and Limitations
The clinical research related to neutropenia and CVC management in AL patients is scarce. The most important advantage of our study is that it was performed in a specific immunocompromised population with an accurate department infection control description in a homogenic sample. This is a retrospective study and a lack of documentation could be possible. However, our electronic medical record reports a systematic description of CVC procedures by nurse team. Multicenter studies are needed to increase the population study, however, due to a medley of clinical research in hematology-oncology patients related to CVC management procedures, is possible to find two departments following the same guidelines recommendations without reports of different CVC procedures (e.g., mechanical valve needleless connectors or split septum connectors, heparin or sodium chloride to CVC lock), and it could be considered a infection control assessment bias [12]. This is a single-center retrospective study. Prospective studies with a multicenter larger size and several homogenic management CVC procedures descriptions should be performed to increase the sample and assess these findings.
Conclusion
We conclude that in neutropenic patiens, undergoing induction therapy or in aplasia support, CLABSI risk increases along with cumulative neutropenia days prior CLABSI and CVC manipulations being the AL patients could be considered a CLABSI risk special population.
Acknowledgment
The authors wish to thank Gillian Ray-Barruel for editing assistance.
References
- Zingg W, Pittet D. Central-line bundles need a multimodal implementation strategy. Lancet Infect Dis. 2016; 16: 631-632.
- Joint Commission, Joint Commission Resources, Inc, & Joint Commission International. Preventing central line-associated bloodstream infections: a global challenge, a global perspective. Joint Commission Resources. 2012.
- Lim MY, Al-Kali A, Ashrani AA, Begna KH, Elliott MA, Hogan WJ, et al. Comparison of complication rates of Hickman® catheters versus peripherally inserted central catheters in patients with acute myeloid leukemia undergoing induction chemotherapy. Leuk Lymphoma. 2013; 54: 1263-1267.
- Chernecky C, Zadinsky J, Macklin D, Maeve MK. The Healthcare and Technology Synergy (HATS) framework for comparative effectiveness research as part of evidence-based practice in vascular access. Journal of the Association for Vascular Access. 2013; 18: 169-174.
- Callister D, Limchaiyawat P, Eells SJ, Miller LG. Risk Factors for Central Line- Associated Bloodstream Infections in the Era of Prevention Bundles. Infect Control Hosp Epidemiol. 2015; 36: 214-216.
- Chopra V, Flanders SA, Saint S, Woller SC, O’grady NP, Safdar N, et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method Michigan Appropriateness Guide for Intravenous Catheters (MAGIC). Ann Intern Med. 2015; 163: S1-S40.
- Zakhour R, Chaftari AM, Raad II. Catheter-related infections in patients with haematological malignancies: novel preventive and therapeutic strategies. Lancet Infect Dis. 2016; 16: e241-e250.
- Ista E, van der Hoven B, Kornelisse RF, van der Starre C, Vos MC, Boersma E, et al. Effectiveness of insertion and maintenance bundles to prevent central–line-associated bloodstream infections in critically ill patients of all ages: a systematic review and meta-analysis. Lancet Infect Dis. 2016; 16: 724-734.
- Baskin JL, Pui CH, Reiss U, Wilimas JA, Metzger ML, Ribeiro RC,et al. Management of occlusion and thrombosis associated with long-term indwelling central venous catheters. Lancet. 2009; 374: 159-169.
- Lukenbill J, Rybicki L, Sekeres MA, Zaman MO, Copelan A, Haddad H, et al. Defining incidence, risk factors, and impact on survival of central line-associated blood stream infections following hematopoietic cell transplantation in acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2016; 19: 720-724.
- Mollee P, Jones M, Stackelroth J, Van Kuilenburg R, Joubert W, Faoagali J, et al. Catheter-associated bloodstream infection incidence and risk factors in adults with cancer: a prospective cohort study. J Hosp Infect. 2011; 78: 26-30.
- Centers for Disease Control and Prevention (CDC). Guidelines for the prevention of intravascular catheter-related infections, 2011. Atlanta: CDC.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: prevention and treatment of cancer-related infections. J NCCN. 2017.
- Chaftari AM, Jordan M, Hachem R, Al Hamal Z, Jiang Y, Yousif A, et al. A clinical practical approach to the surveillance definition of central line– associated bloodstream infection in cancer patients with mucosal barrier injury. Am J Infect Control. 2016; 44: 931-934.
- Gaur AH, Flynn PM, Giannini MA, Shenep JL, Hayden RT. Difference in Time to Detection: A Simple Method to Differentiate Catheter-Related from None- Catheter-Related Bloodstream Infection in Immunocompromised Pediatric Patients. Clin Infect Dis. 2003; 37: 469-475.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 49: 1-45.
- Raad I, Chaftari AM. Advances in Prevention and Management of Central Line- Associated Bloodstream Infections in Patients with Cancer. Clin Infect Dis. 2014; 59: S340-S343.
- Field K, McFarlane C, Cheng AC, Hughes AJ, Jacobs E, Styles K, et al. Incidence of catheter-related bloodstream infection among patients with a needleless, mechanical valve–based intravenous connector in an Australian hematology-oncology unit. Infect Control Hosp Epidemiol. 2007; 28: 610-613.
- Care and maintenance to reduce vascular access complication. Nursing Best Practice Guideline. 2008.
- Mitchell MD, Anderson BJ, Williams K, Umscheid CA. Heparin flushing and other interventions to maintain patency of central venous catheters: a systematic review. J Adv Nurs. 2009; 65: 2007-2021.
- Bradford NK, Edwards RM, Chan RJ. Heparin versus 0.9% sodium chloride intermittent flushing for the prevention of occlusion in long term central venous catheters in infants and children: A systematic review. Int J Nurs Stud. 2016; 59: 51-59.
- Dal Molin A, Clerico M, Baccini M, Guerretta L, Sartorello B, Rasero L. Normal saline versus heparin solution to lock totally implanted venous access devices: Results from a multicenter randomized trial. Eur J Oncol Nurs. 2015; 19: 638-643.
- Abdelkefi A, Achour W, Ben Othman T, Ladeb S, Torjman L, Lakhal A, et al. Use of heparin-coated central venous lines to prevent catheter-related bloodstream infection. J Support Oncol. 2007; 5: 273-278.
- Martinez JM, Leite L, França D, Capela R, Viterbo L, Varajão N. Bundle Approach to Reduce Bloodstream Infections in Neutropenic Hematologic. Acta Med Port. 2015; 28: 474-479.
- Pittiruti M, Bertoglio S, Scoppettuolo G, Biffi R, Lamperti M, Dal Molin A, et al. Evidence-based criteria for the choice and the clinical use of the most appropriate lock solutions for central venous catheters (excluding dialysis catheters): a GAVeCeLT consensus. J Vasc Access. 2016.