
Research Article
Ann Hematol Oncol. 2018; 5(2): 1193.
Determination of Fetomaternal Hemorrhage by Flow Cytometry and Red Blood Cell Alloimmunization in Pregnancy
Kamenicka H¹*, Zajic T², Bydzovska I³ and Prochazkova R1,4
¹Department of Transfusion Medicine, Regional Hospital Liberec, Czech Republic
²Department of Clinical Microbiology and Immunology, Regional Hospital Liberec, Czech Republic
³Department of Gynecology and Obstetrics, Regional Hospital Liberec, Czech Republic
4Faculty of Health Studies, Technical University of Liberec, Czech Republic
*Corresponding author: Kamenicka H, Department of Transfusion Medicine, Regional Hospital Liberec, Baarova 15, 460 63 Liberec, Czech Republic
Received: February 02, 2018; Accepted: March 09, 2018; Published: April 11, 2018
Abstract
Background and Objectives: The aim of the study was the quantitative determination of Fetomaternal Haemorrhage (FMH) by flow cytometry and Red Blood Cell (RBC) antibody identification in pregnancy.
Materials and Methods: We present data from a prospective study of 94 women undergoing obstetric interventions from 12/2013 to 07/2014. FMH by flow cytometry using Anti-HbF, RBC antibody screening and ABO/RhD blood group by serology was determined in peripheral blood anticoagulated blood samples. The RBC antibody screening was repeated six weeks or later in 51 women.
Results: Clinically significant FMH was founded in 9 cases of 94. We present 2 cases of excessive FMH. The first case was a spontaneous vaginal delivery at 40th week of pregnancy with a 4.9% FMH. The second case was acute caesarean section at 40th week of pregnancy with a 6.9% FMH.
8 from 94 samples contained RBC antibodies within the first examination immediately after obstetric procedure. 11 from 51 samples contained RBC antibodies within the second examination.
Conclusion: FMH with possible clinical significance was founded in 9 cases, excessive FMH only twice. We proved the presence of clinically significant and less significant antibodies caused by FMH after the actual or previous pregnancy.
Keywords: Fetomaternal haemorrhage, Alloimmunization, Flow cytometry, Hemolytic disease of the newborn
Introduction
Transplacental transfer of fetal erythrocytes into the maternal circulation is one of complications of pregnancy [1]. When the physiological barrier between the maternal and the fetal circulation is disrupted, the positive pressure gradient may cause fetal erythrocytes to pass into the maternal circulation [2]. Fetomaternal hemorrhage refers to the entry of fetal blood into the maternal bloodstream before or during delivery [3]. FMH occurs normally in minute amounts throughout pregnancy and increases during parturition [4]. Some studies have shown that small bleeds of less than 1 ml of blood occur in 96% of all pregnancies, and that larger losses of approximately 30 ml occur in up to 0.3% of all pregnancies. Although situations are recognized when it is more likely that a large FMH may occur, these often occur without significant signs or symptoms in either the mother or fetus. In many cases the cause of significant FMH remains obscure and is not predictable [1]. Massive FMH may result from a severe pathology such as fetal or maternal trauma or placental defect [5]. During pregnancy or at delivery FMH can cause alloimmunization of the mother, in response to contact with antigens on the surface of fetal red blood cells acquired from the father. The most immunogenic antigens are RhD, followed by c, Kell, E, Kidd and Duffy. Alloimmunization can cause Hemolytic Disease of the Newborn (HDN) when maternal antibodies of IgG-type cross the placenta [6,7]. Sensitized fetal RBCs are destroyed by macrophages in the fetal spleen and by cellular cytotoxicity. HDN happens occasionally in the first pregnancy, but potentially more often in subsequent pregnancies, with a high risk for mortality [6]. Whilst this is known to occur with many blood group antigens historically HDN has most commonly occurred with a RhD negative mother and a RhD positive fetus, with the fetal red cells inducing production of maternal anti-D. The widespread adoption of postpartum immunoprophylaxis with a single dose of RhD immunoglobulin dramatically reduced the incidence of RhD immunization, and HDN [1].
Pregnant women alloimmunization of non-RhD erythrocyte antigens gather importance in conjunction with relative increase of their occurrence [8].
Detection of fetal cells in the maternal circulation during pregnancy is also valuable in the evaluation of fetal welfare following maternal trauma, investigating fetal hydrops or near term fetal death and severe fetal anemia [1].
For the above reasons, a fast and accurate routine test is needed to quantitate fetal RBCs in peripheral maternal blood. Most clinical laboratories perform FMH estimation with the Kleihauer-Betke test [5].
The manual Kleihauer-Betke test for FMH determination is highly sensitive, but its accuracy is in question because of its poor reproducibility, the lack of standardization among laboratories, and the potential sources of error, namely thickness of the blood films, number of RBCs in a low-power microscope field, number of nonstainable fetal cells and variations in pH used [9-13].
Flow cytometry using monoclonal antibodies directed against HbF has some important advantages over the Kleihauer-Betke test in the quantitation of FMH: cytometric methods can accurately distinguish adult F-cells from fetal RBCs; rapidly analyzes a greater number of cells, improving quantitative accuracy; is automated, it has greater reproducibility [14].
The aim of the study was the quantitative determination of FMH by flow cytometry, to report cases of excessive FMH, which was proved by flow cytometry method and identification of unexpected RBC antibodies in pregnancy.
Materials and Methods
Overview of the study
The aim of the study was the quantitative determination of FMH and RBC antibody identification in selected group of women. The RBC antibody screening was repeated six weeks or later after the obstetric procedure to find new alloantibodies caused by FMH after the actual or previous pregnancy.
We present data from the prospective study from 12/2013 to 07/2014 in women after childbirth, abortion, Amniocentesis (AMC) and fetal death. The examination was performed in a Regional Hospital Liberec. FMH, Red cell antibody screening and ABO and RhD blood group was examined in samples of 94 women. All women signed their informed consent to participate in the study and intervention.
The study protocol was approved by Ethical commission of the Regional Hospital Liberec.
Specimen collection and preparation
Mother venous blood samples were collected into an EDTA tube, using aseptic venipuncture. Blood samples were collected 2 hours after the obstetric procedure at Gynecology and Obstetrics Department and stored at either 2-8°C up to 3 days until processing.
Determination of FMH by Flow Cytometry
The fetal Cell CountTM Kit (IQ Products, Groningen, The Netherlands) is intended for the discrimination and quantitative detection of human fetal RBCs in maternal blood. The Fetal Cell CountTM Kit is based on a flow cytometric method, which offers a dual fluorescent detection of two intracellular antigens, Hemoglobin F (HbF) and Carbonic Anhydrase (CA). Both HbF and CA were detected in RBCs obtained from EDTA anti-coagulated mother peripheral whole blood collected 2 hours after the obstetric procedure. Our tests were carried out on the Beckman Coulter Flow Cytometer Cytomics FC 500.The fetal Cell CountTM methodology is based on a combination of two antibodies. One is directed against HbF, which is present in fetal RBCs and in a small percentage of adult RBCs (called F cells). The second antibody is directed against CA, an enzyme only present in adult RBCs and very late stage fetal cells. The dual-color flow cytometric method allows simultaneous detection of these two intracellular antigens, while the use of formaldehyde as fixative and sodium dodecyl sulfate for permeabilization of fixed RBCs results in low background staining, negligible HbF leakage, and minimal cell clumping. Quantification was performed with the Fetal Cell Count kit based on the manufacturer’s procedure, minor changes are indicated in the text.
Half amount of reagents and samples were used for testing. 50 microliters of stored (up to 3 days) blood samples were washed three times with 1 milliliter of Phosphate-Buffered Saline (PBS) before starting the tests.
Fixation and permeabilization
2.5 microliters of RBCs (packed cells) were fixed in the formaldehyde containing solution for 30 minutes at the room temperature. After being washed in PBS, RBCs were permeabilized with the sodium dodecyl sulfate-containing solution for 3 minutes at room temperature. After two washes, RBCs were suspended in 0.5milliliter of PBS.
Immunofluorescent staining
25 microliters of each RBC suspension were incubated for 15 minutes at room temperature (avoiding direct light) with the monoclonal antibody to human fetal hemoglobin conjugated with R-PE and polyclonal antibody to human CA conjugated with FITC.
After final wash in PBS, cells were resuspended in 500 microliters of PBS and were ready for acquisition by flow cytometry.
Data acquisition
List mode files of at least 100,000 events were collected for log FSC, log SSC, and log fluorescence signals for both fluorochrome conjugated antibodies with the region gated at the erythrocytes.
Quality control
FETALtroll was used as an extern control. This is a quality controlled tri-level stabilized blood control with known HbF positive cells content in adult blood (Level 1-normal or negative, Level 2-low positive, Level 3-high positive). The three individual FETALtrol levels were processed in the same way as the patient sample.
Red cell Antibody Screening
The Serascan Diana 4 and the Seracsan Diana 4P (Diagnostic Grifols, S.A., Spain) were intended for the screening of unexpected antibodies, in gel technique DG Gel Neutral/Coombs. The screening of antibodies has the aim of detecting the clinically significant antibodies present in the patient´s sample. The plasma was used for screening of antibodies. Each sample was examined on the fully automated instrument WADiana Compact. Identification of antibodies by manual method was performed in case of a positive antibody screening. 50 microliters of reagent red blood cells were dispensed into the microtubes on DG Gel Neutral/Coombs. 25 microliters of the patient´s plasma were added into the microtubes. Microtubes were incubated at 37°C during 15 minutes, centrifuged in centrifuge for DG gel cards and then were read the results. The RBC antibody screening was repeated six weeks or later after the obstetric procedure to find new alloantibodies.
Quality control
Known positive and negative controls WBcorQC (Immucor, Medizinische Diagnostik GmbH, Germany) were included before each series of screening antibodies.
ABO and RhD Blood Group
Blood grouping reagents ImmuClone anti-A Galileo, ImmuCloneanti-B Galileo, ImmuClone Anti-D rapid Galileo, Novaclone anti-D Galileo, A1 reagent cells, B reagent cells were used for ABO and RhD cell typing on automated microplate tests. All tests were carried out on the fully automated instrument Galileo NEO.
Quality control
Known positive and negative controls WB corQC were included in each series of tests.
Results
Total number of samples was 94. 66 women were after childbirth (52 after vaginal delivery, 14 after caesarean section), 25 after abortion, 2 after amniocentesis and one after fetal death.
Determination of FMH by Flow Cytometry
The value of fetal RBCs diagnosed in maternal circulation ranged from insignificant <0.1% to excessive FMH of 6.9%.Negative FMH <0.1% was present in 73% of cases. Insignificant FMH 0.1-0.25% was present in 17% of cases. Significant or excessive FMH was present in 9.6% (four times vaginal delivery, three times abortion, once caesarean section and once fetal death).Significant FMH exceeding 0.25% was diagnosed after vaginal delivery in 4.3% of all cases, after delivery by caesarean section in 1.1%, after abortion in 3.2% and after fetal death in 1.1%. Excessive FMH was found in 2.1% of cases. Insignificant FMH not more than 0.25% was present in 90.4% of cases. Table 1 shows the quantitative determination of FMH by flow cytometry. Results are divided into 3 groups. Excessive FMH was founded in two cases. We present these 2 cases of clinically excessive FMH.
Group (n = 94)
FMH %
Diagnosis
Week of pregnancy
Group 1 (n = 69)
Negative FMH
< 0.1
Vaginal delivery (n = 35)
Caesarean section (n = 11)
Abortion (n = 21)
Amniocentesis (n = 2)
Group 2 (n = 16)
Insignificant FMH
0.1 – 0.25
Vaginal delivery (n = 13)
Caesarean section (n = 2)
Abortion (n = 1)
Group 3 (n = 9)
Significant FMH
0.26
Abortion
Unknown
Significant FMH
0.32
Abortion
8
Significant FMH
0.44
Vaginal delivery
38
Significant FMH
0.46
Abortion
8
Significant FMH
0.55
Vaginal delivery
41
Significant FMH
0.72
Vaginal delivery
41
Significant FMH
1.06
Fetal death
32
Excessive FMH
4.9
Vaginal delivery
40
Excessive FMH
6.9
Caesarean section
40
Group 1 shows negative values of FMH.
Group 2 shows insignificant values of FMH.
Group 3 shows significant and excessive values of FMH.
Table 1: Results of FMH by flow cytometry.
Case report 1
Spontaneous vaginal delivery (34 year old women) at 40th week of pregnancy with a 4.9% FMH (Figure 1). The blood loss during the delivery was 300 milliliter. There was found no alloimmunization of the mother during pregnancy or after child’s birth. FMH determination was repeated. FMH decreased to 1.9% after 1 week (Figure 2) and to 0.3% after 3 weeks (Figure 3). Excessive FMH did not affect the health of the baby. The newborn had a mild jaundice at the day of hospital discharge (178 μmol/l).
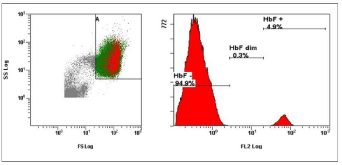
Figure 1: The first examination of FMH 2 hours after the delivery (cytometric
analysis of sample with 4.9% HbF positive fetal cells).
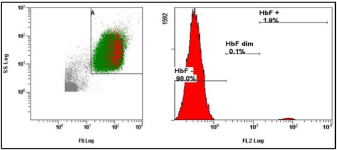
Figure 2: The second examination of FMH 1 week after the delivery (cytometric analysis of sample with 1.9% HbF positive fetal cells).
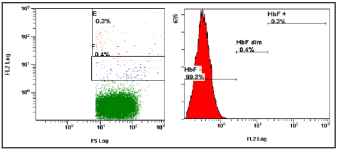
Figure 3: The third examination of FMH 3 weeks after the delivery (cytometric analysis of sample with 0.3% HbF positive fetal cells).
Case report 2
Acute caesarean section (34-year-old women) at 40th week of pregnancy with a 6.9% FMH (Figure 4). The blood loss during the delivery was 300 milliliter. The newborn was significantly anemic. Complete blood count of the newborn was Hgb 20 (g/l), HCT0.07 (l/l), RBC 0.52 (1012/l), MCV 125 (fl), WBC 17.7 (109/l), PLT 80 (109/l). Both mother and child had the same blood type (B RhD positive). There was no alloimmunization of the mother during the pregnancy or after child’s birth. FMH was the reason of child’s anemia and death, but the cause of FMH was hidden.
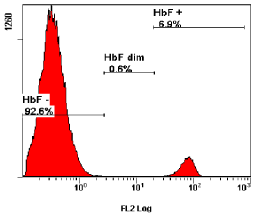
Figure 4: The presented case showing a major peak of HbF negative cells (92.6% maternal cells) and a minor peak of cells containing high levels of HbF, i.e.,
fetal cells (6.9%).
Red Cell Antibody Screening
8 from 94 samples contained RBC antibodies within the first examination immediately after obstetric procedure. The RBC antibody screening of 51 women was repeated and 11 of them contained new antibodies, once anti-Lea and anti-Leb, twice nonspecific antibodies and 8 times anti-D (after IvIg prophylaxis). 43 women did not come to the 2nd examination. Results are shown in Table 2.
The first examination
The second examination
Group
Antibody screening
Antibody identification
Diagnosis
FMH%
Week of pregnancy
Blood loss (ml)
Blood group
Antibody screening
Antibody identification
(n = 94)
Group 1 (n = 8)
Positive
Anti-E
Vaginal delivery
< 0.1
38
200
B RhD positive
Positive
Anti-E,
nonspecifica
Positive
Anti-E
Vaginal delivery
0.15
40
300
0 RhD negative
N.Ib
N.Ib
Positive
Anti-M
AMC
< 0.1
20
Unknown
0 RhD negative
Positive
Anti-M, anti-Dc
Positive
Anti-Cw
Vaginal delivery
< 0.1
37
200
B RhD positive
Positive
Anti-Cw
Positive
Anti-Lea
Caesarean section
< 0.1
40
Unknown
A RhD positive
N.Ib
N.Ib
Positive
Nonspecific, DAT positive, IgG positive, C3d negative
Abortion
< 0.1
Unknown
Unknown
B RhD positive
N.Ib
N.Ib
Positive
Nonspecific
Vaginal delivery
< 0.1
40
250
A RhD positive
N.Ib
N.Ib
Positive
Nonspecific
Vaginal delivery
0.22
41
Unknown
AB RhD positive
N.Ib
N.Ib
Group 2 (n =8 )
Negative
-
Abortion
< 0.1
7
Insignific-ant
A RhD negative
Positive
Anti-Dc
Negative
-
AMC c
< 0.1
18
Unknown
A RhD negative
Positive
Anti-Dc
Negative
-
Vaginal delivery
0,1
41
Insignific-ant
A RhD positive
Positive
Anti-Lea, Anti-Leb
Negative
-
Vaginal delivery
< 0.1
35
200
0 RhD negative
Positive
Anti-Dc
Negative
-
Caesarean section
0.1
38
300
A RhD negative
Positive
Anti-Dc
Negative
-
Vaginal delivery
< 0.1
41
300
A RhD negative
Positive
Anti-Dc
Negative
-
Abortion
< 0.1
7
350
0 RhD negative
Positive
Anti-Dc
Negative
-
Vaginal delivery
0.44
37
250
B RhD negative
Positive
Anti-Dc
Group 3 (n = 78)
Negative (n = 40)
Negative
-
Negative (n = 38)
-
N.Ib
N.Ib
Group 1: 8 samples contained RBC antibodies within the first examination. In 2 of these 8 samples were found new antibodies within the second examination after the obstetric procedure.
Group 2: 8 samples with negative antibody screening within the first examination and positive antibody screening within the second examination after the obstetric procedure.
Group 3: 78 samples with negative antibody screening within the first examination and negative or not investigated antibody screening within the second examination after the obstetric procedure.
Table 2: Immunohematology results of 94 samples.
Discussion
The aim of this prospective study was the quantitative determination of FMH by flow cytometry and identification of unexpected RBC antibodies in pregnancy. FMH and RBC screening was examined in the sample of 94 women. 66 women were after childbirth, 25 after abortion, 2 after amniocentesis and one after fetal death.
In our experiment we chose a kit that uses anti-HbF and anti-CA antibodies. The volume of FMH during vaginal delivery ranged from <0.1% to 4.9%, delivery by caesarean section ranged from <0.1% to 6.9%, abortion ranged from <0.1% to 0.46%, FMH of fetal death was 1.06%. In our study, significant or excessive amounts of FMH were detectable in 9.6% of patients by flow cytometry method in pre or postdelivery samples. Significant FMH exceeding 0.25% was diagnosed after vaginal delivery in 4.3% of all cases, after delivery by caesarean section in 1.1%, after abortion in 3.2% and after fetal death in 1.1%. Excessive FMH was found in 2.1% of cases. Immunohematologic cause of excessive FMH has not been proven. Insignificant FMH not more than 0.25% was present in 90.4% of cases.
We proved the presence of clinically significant and less significant antibodies caused by FMH after the actual or previous pregnancy. 8.5% samples contained RBC antibodies within the first examination immediately after obstetric procedure (two times anti-E, once anti-M, once anti-Cw, once anti-Lea and three times nonspecific antibodies). The second examination was performed in a group of 51 women, other women were absent for examination. 21.6% of examined women samples contained new antibodies, once anti-Lea and anti-Leb, twice nonspecific antibodies and 8 times anti-D (after IvIg prophylaxis).
Prophylactic anti-D administration prevents significantly Rhesus D alloimmunization and reduces the risk of hemolytic disease of the fetus and newborn. Pregnant women alloimmunization of non-RhD erythrocyte antigens gather importance in conjunction with relative increase of their occurrence. Although these erythrocyte antigens are able to induce antibody response in mother and result in subsequent hemolytic disease of fetus and newborn, prophylaxis is not possible.
Detection of fetal cells in maternal circulation represents an important support for the clinical diagnosis of FMH. Clinicians may wish to apply this rapid and accurate method for diagnostic purposes postnatally and even antenatally when clinical suspicion arises.
Acknowledgement
This work was supported by a Research department of the Regional Hospital Liberec (VR 130310). We thank workers from Department of Transfusion Medicine and Department of Gynecology and Obstetrics for their technical assistance.
References
- Scientific subcommittee of the Australian & New Zeland Society of Blood Transfusion INC. Guidelines for laboratory assessment of fetomaternal haemorrhage. Sydney: The Society. 2002; ISBN No: 0957726228.
- Lubušký M, Studnicková M, Procházka M. Fetomaternální hemoragie Postgraduální medicína. 2012; 14: 282-289.
- Ruffini E, Bianchi AM, De Petris L, Fares MK, Zorzi G, Carlucci A. Chronic Massive Fetomaternal Hemorrhage in a newborn from immigrants. Clinical and organizational implications. Pediatr Med Chir. 2012; 34: 241-243.
- Wong L, Hunsberger BC, Bruce Bagwell C, Davis BH, et al. Automated quantitation of fetomaternal hemorrhage by flow cytometry for HbF-containing fetal red blood cells using probability state modeling. Int J Lab Hemato0l. 2013; 35: 548-554.
- Porra V, Bernaud J, Gueret P, Bricca P, Rigal D, Follea G, et al. Identification and quantification of fetal red blood cells in maternal blood by a dual-color flowcytometric method: Evaluation of the Fetal Cell Count kit. Transfusion. 2007; 47: 1281-1289.
- Agarwal P, Sekhar Das S, Gupta R, Khetan D, Chaudhary R. Quantification of Feto-Maternal Hemorrhage: Selection of Techniques for a Resource-Poor Setting. Gynecol Obstet Invest. 2011; 71: 47-52.
- Gielezyska A, Fabijanska-Mitek J, Debska M. Detection of fetomaternal haemorrhage using the microcolumnagglutination test. Post N Med. 2016; XXIX: 97-99.
- Petroš M, Lubušký M, Šimetka O, Procházka M. Aloimunizace têhotných žen non-RhDerytrocytárními antigeny: prehledový çlánek. Ces. Gynek. 2010; 75: 325-333.
- Ben-Haroush A, Belkin A, Chezar J, Orlin J, Hod M, Bar J. Comparison of two techniques for the evaluation of fetomaternal hemorrhage in RhDnegative women: Gel agglutination and haemoglobin F determination by flowcytometry. Acta Obstet Gynecol Scand. 2007; 86: 821-826.
- Radel DJ, Penz CS, Dietz AB, Gastineau DA. A combined flowcytometrybased method for fetomaternal hemorrhage and maternal D. Transfusion. 2008; 48: 1886-1891.
- Savithrisowmya S, Singh M, Kriplani A, Agarwal N, Mehra NK, Bhatla N. Assessment of fetomaternal hemorrhage by flow cytometry and kleihauerbetke test in rh-negative pregnancies. Gynecol Obstet Invest. 2008; 65: 84- 88.
- Peedin AR, Mazepa MA, Park YA, Weimer ET, Schmitz JL, Raval JS. Two cases of asymptomatic massive fetomaternal hemorrhage. Transfus Apher Sci. 2015; 52: 208-210.
- Tsafrir A, Abramov Y, Preminger A, Ezra Y, Fibach E. Severe fetomaternal hemorrhage confirmed and quantified by flow cytometry using anti fetal hemoglobin antibodies. Acta Obstet Gynecol Scand. 2002; 81: 364-365.
- Kim, YA, Makar RS. Detection of fetomaternal hemorrhage. Am J Hematol. 2012; 87: 417-423.