
Case Report
Ann Hematol Oncol. 2018; 5(3): 1197.
Nocardiosis Following Allogeneic Stem Cell Transplantation
Xavier Roussel¹*, Ana Berceanu¹, Etienne Daguindau¹,², Yohan Desbrosses¹, Eric Deconinck¹,² and Fabrice Larosa¹
¹University Hospital of Besancon, Department of Hematology, F-25000 Besançon, France
²University of Bourgogne Franche Comté, INSERM, EFS BFC, UMR 1098, F-25000 Besançon, France
*Corresponding author: Xavier Roussel, Hematology Department, CHU Besancon, Hospital Jean Minjoz, 1 Bld Fleming F-25000, Besancon, France
Received: March 16, 2018; Accepted: April 17, 2018; Published: May 04, 2018
Abstract
Nocardiosis after Hematopoietic allogeneic stem cell transplant (HSCT) is rare and is known to be particularly severe. We described two cases of nocardiosis after HSCT, one with localized pulmonary disease and pneumocystisis co-infection and the other one with cerebral localization and exposed our clinical and diagnosis management. In these two brief reports, we proved that nocardiosis may rarely be developed in the first months after HSCT. Diagnosis remains difficult and clinical signs are currently none specific. CT scan can help to suspect diagnosis. Biopsy is the gold standard diagnosis method to prove nocardiosis with culture but frequently not available. Early diagnosis could prevent clinical dissemination or pulmonary complication with prompt initiation of adapted anti-nocardial antibiotic including penem-based regimen. Moreover, a poor immune reconstitution particularly lack of thymic function is also determinant after HSCT. TMP-SMX remains the most effective antibiotic curative and prophylactic therapeutic for Nocardiosis.
Keywords: Nocardiosis after Hematopoietic allogeneic stem cell transplant (HSCT); Localized pulmonary nocardiosis; Cerebral extended nocardiosis; Trimethoprim-sulfamethoxazole nocardiosis prophylaxis; Nocardiosis PCR; Immune CD4+ reconstitution
Introduction
Nocardiosis after Hematopoietic allogeneic stem cell transplant (HSCT) is rare, with incidence of 1.99 % reported among our center at Besancon university-hospital [1], but known to be particularly severe with related-mortality rate range between 7 to 44% [2,3] and sometimes more (77%) [4]. Several cases of nocardiosis were described, with localized pulmonary disease, the primary infectious site (71%) and cerebral extended disease, the most common disseminated infectious site [3]. Diagnosis of nocardia was defined as positive PCR replication or culture [5,6], and concordant CT scan iconography [7]. We described two cases of nocardiosis after HSCT, one with localized pulmonary disease and pneumocystisis coinfection and the other one with cerebral localization and exposed our clinical and diagnosis management.
Case Presentation
Case 1
A 58-years old man, with acute myeloid leukemia (AML) in first complete response with unfavorable cytogenetic prognostic received a sibling 12/12 peripheral blood stem cell (PBSC) allogeneic stem cell transplant (HSCT) in October 2015. We performed sequential conditioning regimen including fludarabine 30 mg/ m² from D-13 to D-10, cytarabine 2 g/m² from D-13 to D-10, amsacrine 100mg/m² from D-13 to D-10, busulfan 3.2 mg/kg from D-6 and D-5, cyclophosphamide 50 mg/kg at D-4. Graft infusion (5.6x106 CD34+-cells/kg) was performed on D+0 of conditioning regimen. Graft-versus-Host disease (GVHD) prophylaxis consisted of antithymoglobuline serum (ATG) 2.5 mg/kg/day D-2 and D-1, cyclosporine A (CsA) IV 3 mg/kg/day at D-2 then adapted to blood level and mycophenolate mofetil 30 mg/kg D-2. CsA was switched to tacrolimus for cutaneous grade 2 acute GVHD (aGVHD) at D+14. CMV replication occurred at D+ 40 and was controlled with valgancyclovir (900 mg twice daily for 14 days) (donor and recipient CMV match D+/R+). No improvement of skin aGVHD was observed and JAK1/2 inhibitor (ruxolitinib) 5 mg twice daily and 1 mg/kg prednisone was successfully introduced at D+77 (Figure 1A and 1B).
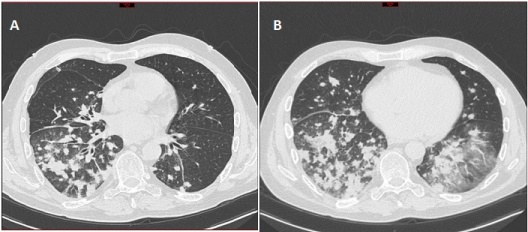
Figure 1:
Three months after HSCT, the patient developed a persistent cough, hypoxemia and fever. No pulmonary embolism was found in CT scan but multiple pulmonary parenchymal nodules, patchy consolidation, ground-glass opacity in the 2 lung fields and mediastinal lymphadenopathy (picture n°1). Sputum culture, aspergillus platellia antigen, blood quantitative CMV PCR, blood toxoplasmocytosis PCR, aspergillosis and mucormycosis PCR were negative. Bronchoscopy with bronchoalveolar lavage confirmed Nocardia absessus by PCR positivity and proved by culture and Pneumocystis jirovecii by PCR. Induction antibiotherapy was started with imipenem (1 g 3 times daily) and trimethoprim-sulfamethoxazole 4800mg/960mg daily (TMP-SMX) based-regimen. Empirical liposomal amphotericin B therapy was stopped after documentation of infections. Clinical and radiological signs were promptly improved in one week. Cerebral MRI was negative. TMP-SMX observance was doubtful after novel patient interrogation. After an initial 30 days of imipenem based-therapy, ceftriaxone (2 g daily) was associated to TMP-SMX P.O. 1 tablet (800 mg/160 mg) 3 times weekly up to 180 days. Long-term secondary prophylaxis with TMP-SMX 1 tablet (800 mg/160 mg) 3 times weekly was applied without nocardiosis relapse. Complete donorchimerism was obtained at nocardiosis onset and was persistent 3 months after nocardiosis. Patient was still in remission at onset and after nocardiosis resolution. Lymphopenia and particularly low CD4+ T-cells count (75/μL) was assessed at diagnosis. Lack of CD4+ T-cells recovery (298/μL) was still noted 7 months after nocardiosis, and especially naïve CD4+ T-cells with 27/μL CD4+CD45RA+/RO- T cells count.
Case 2
A 47-years old women, with AML in first complete received in April 2015 a myeloablative non-related 10/10 HLA-matched bone marrow HSCT (2.45x106 CD34+/kg) after fludarabine 40 mg/m²/ day from D-6 to D-3 and busulfan IV 3.2 mg/kg/day from D-6 to D-3 conditioning regimen. GVHD prophylaxis included ATG 2.5 mg/kg/ at D- and D-2 , CsA IV 3 mg/kg/day at D-2 then adapted to blood level and short methotrexate regimen 15 mg/m² D+1 and 10 mg/m² at D+3 and D+6. Skin aGVHD grade 2 was first treated with 1.5mg/kg prednisone. CsA was switched to tacrolimus at D+64 graft infusion. We performed weekly a total of four injections of rituximab (375 mg/m²/infusion) for PCR EBV detection (40000 copies/μL) and to reduce aGvHD. TMP-SMX was withdrawn in May 2015 due to hematological G2-3 toxicity.
Nine months after HSCT, the patient developed pulmonary failure with neurological confusion without improvement after oral empiric broad spectrum antibiotherapy (amoxicillin-clavulanic acid 1 g/125 mg three times daily and ciprofloxacine 400 mg twice daily for a total of 3 days. X-ray chest radiography, cerebral CT scan and MRI performed initially concluded to lobar consolidation and cerebellum abscess (picture n° 2a). Thoracic CT scan found right superior lobar abscess with cavitation, atelectasis, multiple perivascular pulmonary micro nodules, lymphadenopathy, patchy consolidation, ground-glass opacity, and bilateral pleural effusion (picture n° 2bc). Complete biological screening: lumbar puncture with cerebral spinal fluid culture, sputum culture, nasopharyngeal lavage, CMV and toxoplasmocytosis whole blood quantitative PCR, aspergillosis platelia antigen and mucormycosis PCR was negative. Bronchoscopy with bronchoalveolar lavage culture and PCR confirmed Nocardia nova documentation. Firstly, meropenem (2 g 3 times daily) and linezolid IV 600 mg x2/day were introduced with rapid neurological and pulmonary improvement. This double antibiotherapy was maintained during 180 days and then switched to cefotaxime (2 g 3 times daily) and TMP-SMX PO 1 tablet (800 mg/160 mg) 3 times weekly for a total of 360 days. No further prophylactic SMP-SMX treatment was continued (Figure 2A, 2B and 2C).
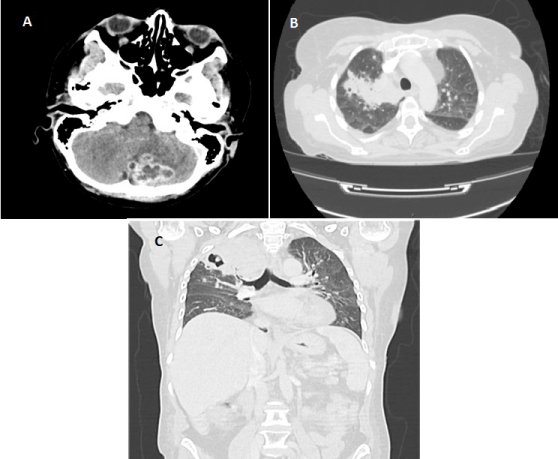
Figure 2:
Two months after antibiotherapy ending, the patient presented febrile headache and meningitis signs. Lumbar puncture with cerebral spinal fluid culture confirmed nocardiosis relapse. Cerebral MRI showed leptomeningitis with T1 gadolinium and T2 hyperintense signal without abscess in this second episode. Initial antibiotic with meropenem (2 g 3 times daily), linezolid (600 mg twice daily) and amikacin (15 mg/kg for 5 days) was started. After 180 days of treatment, a consolidation treatment with meropenem (2 g 3 times daily) and minocycline (200 mg twice daily) was continued for a total of 360 days, then maintenance by TMP-SMX 1 tablet (800mg/160mg) 3 times daily was still ongoing. Complete chimerism was obtained at nocardiosis and persistent during bacterial infection. The patient was still in complete remission of the underlying hematological disease during all nocardiosis treatment. After the end of the second antibiotic regimen, cerebral nocardiosis was confirmed to be cure. No further GVHD relapse was observed subsequently. Cellular adaptive immune response still remained deficient with only CD4+ T-cells count at 277/μL 12 months after nocardiosis relapse, and especially naïve CD4+ T-cells with 85/μL CD4+CD45+RA+/RO- T cells count.
Discussion
Nocardiosis after Hematopoietic allogeneic stem cell transplant (HSCT) is rare, with incidence of 1.99% reported among our center at Besancon university-hospital [1], but known to be particularly severe with related-mortality rate range between 7 to 44% [2,3] and sometimes more (77%) [4]. Several cases of nocardiosis were described, with localized pulmonary disease, the primary infectious site (71%) and cerebral extended disease, the most common disseminated infectious site [3]. Diagnosis of nocardia was defined as positive PCR replication or culture [5,6], and concordant CT scan iconography [7].
In these two brief reports, we proved that nocardiosis may rarely be developed in the first months after HSCT. Nocardial–related mortality may be important about 50% [8]. Lung is the most common involvement in nocardiosis through respiratory tract infestation [9]. Central nervous system is the frequent disseminated infectious site [3]. Diagnosis remains difficult and clinical signs are currently none specific. CT scan can help to suspect diagnosis [7]. Biopsy is the gold standard diagnosis method to prove nocardiosis with culture but frequently not available. Of interest nocardial PCR assessment is now admitted to be unavoidable in diagnosis with appropriate sensibility and specificity [5,10]. Early diagnosis could prevent clinical dissemination or pulmonary complication with prompt initiation of adapted anti-nocardial antibiotic including penem-based regimen [8] in order to decrease this poor prognosis. After HSCT, delayed immune recovery and large use of immunosuppressive agents (CsA and high dose of steroids) in GHVD treatment are major contributing factors described to increase risk of Nocardia infection [1,11,12]. Indeed, a poor immune reconstitution particularly lack of thymic function is also determinant after HSCT [1,13-15]. CMV reactivation and other associated opportunist infections were also described as well to be implicated [8,14,16]. TMP-SMX remains the most effective antibiotic curative and prophylactic therapeutic [8,9]. Nevertheless, most patient developed nocardiosis during TMP-SMX free periods. This remains probably the major risk factor of nocardiosis especially as resistance to TMP-SMX is rare [17]. TMP-SMX prophylaxis should be maintained until immune reconstitution especially naïve CD4+ T-cell (CD4+CD45RA+/RO-) recovery with broad repertoire derived from thymic out [1] and withdrawal of immunosuppressive medication.
References
- Mansi L, Daguindau E, Saas P, Pouthier F, Ferrand C, Dormoy A, et al. Diagnosis and management of nocardiosis after bone marrow stem cell transplantation in adults: Lack of lymphocyte recovery as a major contributing factor. Pathol Biol. 2014; 62: 156-161.
- Cattaneo C, Antoniazzi F, Caira M, Castagnola C, Delia M, Tumbarello M, et al. Nocardia spp infections among hematological patients: results of a retrospective multicenter study. Int J Infect Dis. 2013; 17: e610-4.
- Ambrosioni J, Lew D, Garbino J. Nocardiosis: Updated Clinical Review and Experience at a Tertiary Center. Infection. 2010; 38: 89-97.
- Husain S, McCurry K, Dauber J, Singh N, Kusne S. Nocardia infection in lung transplant recipients. J Heart Lung Transplant. 2002; 21: 354-359.
- Ashdown LR. An improved screening technique for isolation of Nocardia species from sputum specimens. Pathology (Phila). 1990; 22: 157-161.
- Steingrube VA, Brown BA, Gibson JL, Wilson RW, Brown J, Blacklock Z, et al. DNA amplification and restriction endonuclease analysis for differentiation of 12 species and taxa of Nocardia, including recognition of four new taxa within the Nocardia asteroides complex. J Clin Microbiol. 1995; 33: 3096- 3101.
- Orlowski HLP, McWilliams S, Mellnick VM, Bhalla S, Lubner MG, Pickhardt PJ, et al. Imaging Spectrum of Invasive Fungal and Fungal-like Infections. Radiogr Rev Publ Radiol Soc N Am Inc. 2017; 37: 1119-1134.
- Lebeaux D, Morelon E, Suarez F, Lanternier F, Scemla A, Frange P, et al. Nocardiosis in transplant recipients. Eur J Clin Microbiol Infect Dis. 2014; 33: 689-702.
- Chen J, Zhou H, Xu P, Zhang P, Ma S, Zhou J. Clinical and Radiographic Characteristics of Pulmonary Nocardiosis: Clues to Earlier Diagnosis. PLOS ONE. 2014; 9: e90724.
- Rodríguez-Nava V, Couble A, Devulder G, Flandrois J-P, Boiron P, Laurent F. Use of PCR-Restriction Enzyme Pattern Analysis and Sequencing Database for hsp65 Gene-Based Identification of Nocardia Species. J Clin Microbiol. 2006; 44: 536-546.
- Arduino RC, Johnson PC, Miranda braham G. Nocardiosis in Renal Transplant Recipients Undergoing Immunosuppression with Cyclosporine. Clin Infect Dis. 1993; 16: 505-512.
- Wilson JW. Nocardiosis: Updates and Clinical Overview. Mayo Clin Proc. 2012; 87: 403-4077.
- Thiel A, Alexander T, Schmidt CA, Przybylski GK, Kimmig S, Kohler S, et al. Direct Assessment of Thymic Reactivation after Autologous Stem Cell Transplantation. Acta Haematol. 2008; 119: 22-27.
- Geddes M, Storek J. Immune reconstitution following hematopoietic stem-cell transplantation. Best Pract Res Clin Haematol. 2007; 20: 329-348.
- Deem RL, Doughty FA, Beaman BL. Immunologically specific direct T lymphocyte-mediated killing of Nocardia asteroides. J Immunol. 1983; 130: 2401-2406.
- Minero MV, Marín M, Cercenado E, Rabadán PM, Bouza E, Muñoz P. Nocardiosis at the Turn of the Century. Medicine (Baltimore). 2009; 88: 250.
- Schlaberg R, Fisher MA, Hanson KE. Susceptibility Profiles of Nocardia Isolates Based on Current Taxonomy. Antimicrob Agents Chemother. 2014; 58: 795-800.