
Case Report
Ann Hematol Oncol. 2018; 5(4): 1204.
A Case of Unresectable Basal Cell Carcinoma with Response to Novel Hedgehog Inhibitor Therapy
Mabanta S1*, Maddukuri M1 and Miksits D2
1Department of Radiation Oncology, Cancer Center at Wise Health System, USA
2Department of Pathology, Wise Health System, USA
*Corresponding author: Mabanta S, Department of Radiation Oncology, Cancer Center at Wise Health System, USA and Association with Choice Cancer Care, USA
Received: April 17, 2018; Accepted: May 07, 2018; Published: May 22, 2018
Abstract
Basal cell carcinoma (BCC) is the most common cancer worldwide. Traditional treatments include surgery and radiation. We present a case of locally advanced BCC with an excellent response to a combination of Hedgehog inhibitor therapy and radiation.
A 70 year old gentleman, presented with a disfiguring mass lesion on the nose and cheeks, which had progressively worsened for seven years prior to presentation.The disease was eroding the nose, cheeks, upper lip, and lower eyelids. A biopsy from the left cheek revealed Basal cell carcinoma. His disease was felt unResectable and hence was started on Hedgehog inhibitor therapy, Vismodegib (Erivedge). He tolerated it well with near complete response to the treatment. Subsequently, he had radiation therapy to the affected area and is currently doing well.
Vismodegib and Sonidegib, approved now for treatment of locally advanced and metastatic basal cell carcinoma, appear very promising and are changing the paradigm of treatment of this disease. Their efficacy is also being looked at in other solid tumors.
Keywords: Basal cell carcinoma; Hedgehog inhibitors; Vismodegib
Abbreviations
BCC: Basal Cell Carcinoma; laBCC: Locally Advance Basal Cell Carcinoma; mBCC: Metastatic Basal Cell Carcinoma; HH: Hedgehog
Case Presentation
A 70-year-old gentleman has self-referred to us for a mass on his nose. Initially the mass was small at the tip of his nose, but unfortunately, he let it grow for seven years before seeking medical care. On clinical exam, the tip of the nose had eroded as well as the majority of the left side of the nose, extending onto the bilateral cheeks, into the inferior eyelids, and into the bilateral lacrimal glands. The mass which measured approximately 8x10 cm also extended into the upper lip. The patient complained of increased tear formation but denied pain or epistaxis. He was a smoker (one pack a day for 50 years), a social alcohol user, and a truck driver by occupation. His left cheek biopsy showed basal cell carcinoma (Figure 1,2). A CT of the neck showed no pathologic lymphadenopathy. CT sinuses showed nasal and paranasal abnormal and irregular contour. Diffuse nasal cavity, maxillary, and frontal sinus mucosal thickening was noted. Clinically, he had T4N0 basal cell carcinoma of the nose/cheek (Figure 3). Due to the un-resectable and locally advanced stage of his cancer, he was started on neoadjuvant hedgehog inhibitor therapy, Vismodegib (Erivedge), for 5 months. He achieved near complete tumor response. He tolerated the Erivedge well with no untoward side effects (Figure 4). He subsequently completed a course of radiation therapy to the nose and cheek. He received 6000cGy/30 fractions, 6MV, and using 3D conformal technique with lateral fields and 0.5 cm bolus on the skin. He tolerated radiation well except for minor nosebleeds, skin erythema and skin tenderness in the radiated field. In follow-up, the patient continues to do well. He has no obvious recurrence six months after completion of radiation treatment.
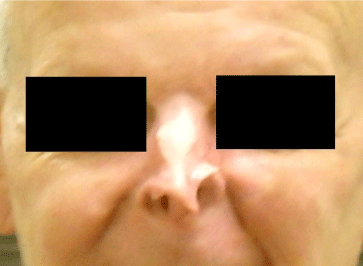
Figure 4: 5 months after initiation of Vismodegib treatment.
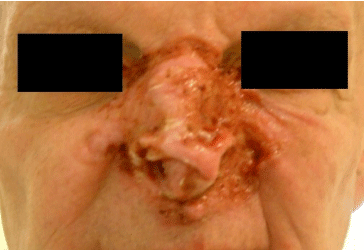
Figure 3: Basal cell carcinoma of the nose, at presentation, prior to initiation of Vismodegib treatment.
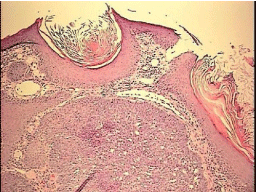
Figure 1: L. cheek lesion, 10X magnification, H&E stain. Basal cell carcinoma showing infiltrating. Basaloid cells with peripheral palisading.
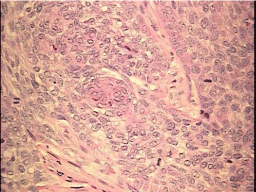
Figure 2: L. cheek lesion, 40X magnification, H&E stain. Basal cell carcinoma with focal squamous differentiation and frequent mitotic figures.
Discussion
Basal cell carcinoma is the most common type of skin cancer diagnosed in the world. Its incidence worldwide continues to rise with more than 2 million people diagnosed every year in United States alone.
Risk factors include Ultraviolet radiation exposure [1], use of tanning beds, [2] host factors such as eye and hair color, skin type, and immunosuppressed states seen in conditions like organ transplantation and HIV, as well as in genetic disorders like Gorlin syndrome, Xeroderma pigmentosum and albinism [3-5].
Basal cell carcinomas are generally slow growing and rarely metastasize and have good prognosis. BCC related mortality is very small, but due to very high incidence it does carry significant morbidity, quality of life issues, and financial burden [6].
Surgical excision is the gold standard for small, early stage, localized BCC. It can be simple excision orMohs micrographic surgery. Other treatment options include curettage and electrodessication, superficial field therapies like 5-Fluorouracil, imiquimod, photodynamic therapy, and primary or adjuvant radiation therapy [7].
Advanced BCC is divided into two categories: locally advanced (laBCCs) and metastatic (mBCCs). laBCC s include primary tumors that invade and extend beyond the skin into the cartilage, muscles, and bone or have spread to the skin and/or lymph nodes in the immediate vicinity of the primary site. Metastatic BCC involves distant spread to another organ or no regional lymph nodes/skin and is very rare [8]. Surgery and radiation play a role in advanced disease also and may cause significant disfigurement and morbidity. Until recently,the role of systemic therapy for advanced BCC was very limited. However, the development of hedgehog pathway inhibitors has dramatically changed the paradigm of systemic treatment of laBCCs and mBCCs.
The Hedgehog (HH) gene was discovered in 1980 by Nusslein- Volhard and Wieschaus through genetic analysis of the fruit fly Drosophila melanogaster [9]. In the early 1990s, three HH gene homologues were discovered in vertebrates: Sonic hedgehog (Shh), Indian hedgehog (Ihh), and Desert hedgehog (Dhh). Shh is the most potent of these ligands and plays an essential role in embryonic development and is critical in maintaining tissue polarity [10]. The Hh pathway is tightly regulated in adults and remains quiescent. In the absence of the Hh ligand signal, the cell surface transmembrane protein PTCH-1 keeps the activity of the 7-membrane-spanning receptor, SMO, suppressed [11]. However, when the Hh signaling ligand is present and binds to PTCH-1, SMO is no longer inhibited and allows the transcription factor Gli to enter the nucleus of the cell, promoting cell division and tumor genesis [12]. The abnormal Hh signaling due to mutations in the Hh pathway has been implicated in the pathogenesis of sporadic basal cell carcinomas [13]. The discovery of cyclopamine, a natural alkaloid product known to bind to SMO protein, resulting in inhibition of Hh signaling, opened new gates for the research of Hh pathway inhibitors in cancer treatment [14].
another organ or no regional lymph nodes/skin and is very rare [8]. Surgery and radiation play favorable pharmaceutical properties compared to Cyclopamine. A phase I study of GDC- 0449 in 2009 showed a 58% response rate among 33 patients with laBcc and mBcc [15]. Approval of Vimodegib 150mg once a day for laBcc and mBcc in Jan 2012 was based on the ERIVANCE clinical trial, a multicenter, international, two-cohort, nonrandomized study [16]. The primary endpoint of this trial was the objective response rate. RECIST criteria were used to assess response in mBCC. In 63 patients with laBCC, the ORR, including both complete and partial response, was 43%, with complete responses in 13 patients (21%). In 33 patients with mBCC, the ORR was 30% and all responses were partial. 24 patients had stable disease and 8 patients had progressive disease [16]. The median duration of response was 7.6 months and the median progression free survival was 9.5 months. A follow-up 21 month analysis of ERIVANCEtrial confirmed the efficacy and safety of Vismodegib in advanced BCC [17]. The ORR increased from 43% to 47.6% in the laBCC and from 30% to 33.3% in patients with mBCC. The median duration of response in patients with laBCC increased from 7.6 months to 9.5 months [17]. Adverse events occurring in more than 20% of patients were muscle spasms (68%), alopecia (63%), dysgeusia (51%), weight loss (46%), fatigue (36%), nausea (29%), decrease in appetite (23%), and diarrhea (22%). Grade 3 or 4 adverse events included muscle spasms, weight loss, fatigue, and loss of appetite.
STEVIE (Clinical trials.gov, NCT01367665) is an ongoing international, single-arm, open-label study for Vismodegib in laBCC or mBCC accruing patients with safety as the primary objective. The primary analysis demonstates the tolerability of Vismodegib and the safety profile is consistent with the previous studies.
LDE225 (Sonidegib), another Hh signaling pathway inhibitor, was FDA approved in July 2015 for the treatment of adult patients with laBCC who are not candidates for surgery or radiation therapy, as well as for patients who have recurred following surgery or radiation therapy. Approval was based on the efficacy and safety from the BOLT trial, an international, randomized, double-blind, Phase II study [18]. Patients were randomized to receive 1:2 into the Sonedegib 800 mg and 200 mg QD treatment arms. ORR in the 200mg and 800 mg arms were 47% and 35.2% in laBCC and 15.4% and 17.4% in mBCC respectively. The most common adverse effects included muscle spasms, alopecia, dysgeusia, nausea, elevated blood CK, and fatigue. Although both groups demonstrated similar efficacy, the 200mg daily dose group had longer duration of treatment, lower rate of adverse effects, and lower discontinuation rates. In the BOLT 12 month analysis, Sonidegib continued to demonstrate sustained responses with no new safety concerns.
Most patients with advanced basal cell carcinoma achieve disease control with hedgehog signaling pathway inhibitors. However, some patients are intrinsically resistant to treatment and some others acquire resistance during treatment. Genomic analysis of tumor biopsies showed that Vismodegib resistance is associated with Hh pathway reactivation, mainly caused by SMO mutations and, to a lesser extent, through mutations in suppressor of fused (SUFU) and gain of function mutations in Gli2 [19]. A combination of therapies targeting both SMO and downstream components of the Hh pathway may be a potential alternative strategy to overcome drug resistance.
References
- Gallaghar RP, Hill GB, Bajdik CD, Finchman S, Coldman AJ, Mc lean DI, et al. Sunlight exposure, pigmentary factors and risk of nonmelanocyte skin cancer. I. Basal cell carcinoma. Arch Dermatol. 1995; 131: 157-163.
- Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012; 30: 1588-1593.
- Wilkins K, Turner R, Dolev JC, LeBoit PE, Berger TG, Maurer TA. Cutaneous malignancy and human immunodeficiency virus disease. J Am Acad Dermatol. 2006: 54: 189-206.
- Tessari G, Girolomoni G. Nonmelanoma skin cancer in solid organ transplant recipients: update on epidemiology, risk factors and management. Derm Surg. 2012; 38: 1622-1630.
- Castori M,Morrone A, Kanitakis J,Grammatico P. Genetic skin diseases predisposing to basal cell carcinoma. Eur J Dermatol. 2012; 22: 299-309.
- Cakiro BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plassurg Clin North Am. 2012; 20: 419- 422.
- Kauvar AN, Cronin T Jr, Roenigk R, Hruza G, Bennett R. American Society for Dermatologic Surgery. Consensus for nonmelanoma skin cancer treatment: basal cell carcinoma, including a cost analysis of treatment methods. DermatolSurg. 2015; 41: 550-571.
- Wysong A,Aasi SZ, Tang JY. Update on metastatic basal cell carcinoma: a summary of published cases from 1981 through 2011. JAMA Dermatol. 2013; 149: 615-616.
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in drosophila. Nature. 1980; 287: 795-801.
- Pathi S, Pagan-WestphalS, Baker DP, Garber EA, Rayhorn P, Bumcrot D, et al. Comparative biological responses to human Sonic, Indian, and Desert hedgehog. Mech. Dev. 2001; 106: 107-117.
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, et al. The tumour suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996; 384: 129-134.
- Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J,Bonifas JM, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and patients with the basal cell nevus syndrome. J Invest Dermatol. 1998; 110: 885-888
- Reifenberger J, Wolter M, Knobbe CB, Kohler B,Schonicke A, schwarwachter C, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol. 2005; 152: 43-51.
- Gould A, Missailidis S. Targeting the hedgehog pathway: the development of cyclopamine and the development of anti-cancer drugs targeting the hedgehog pathway. Mini Rev Med Chem. 2011; 11: 200-213.
- Von Hoff DD,LoRusso PM, Rudin CM, Reddy JC,Yauch RL, Tibes R, et al. Inhibition of the Hedgehog pathway in advanced basal- cell carcinoma. N Engl J Med. 2009. 361; 1164-1172.
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, et al. Efficacy and safety of vismodegib in advanced basal cell carcinoma. The New Engl J Med. 2012; 366: 2171-2179.
- Sekulic A, Migden MR, Lewis K, Hainsworth JD, Solomon JA, Yoo S, et al. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermato. 2015: 72: 1021-1026.
- Migden MR, Guminski A, Gutzmer R, Dirix L, Lewis KD, Combemale P, et al. Treatment with two different doses of Sonidegib in patients with locally advanced or metastatic basal cell carcinoma (BOLT): a multicenter, randomized, double-blind phase 2 trial. Lancet Oncol. 2015. 16: 716-728.
- Sharpe HJ, Pau G, Dijkgraaf GJ, Basset-Seguin N, Modrusau Z, Sarink Y, et al. Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell. 2015; 27: 327-341.