
Research Article
Ann Hematol Oncol. 2018; 5(5): 1207.
BCL2/IGH Chromosomal Translocation t(14;18) is not Common in Lymphocytes of Healthy Individuals in the Southern Part of Iran
Dehghani M1, Monabati A2, Valibeigi B3 and Derakhshan A4*
1Department of Internal Medicine and Hematology/ Oncology, Hematology Research Center, Shiraz University of Medical Sciences, Iran
2Department of Pathology and Hematology Research Center, Shiraz University of Medical Sciences, Iran
3Department of Pathology, Molecular Pathology Ward, Shiraz University of Medical Sciences, Iran
4Medical Genetic Lab, Shiraz Infertility Treatment Center, Iran
*Corresponding author: Alireza Derakhshan, Medical Genetic Lab, Shiraz Infertility Treatment Center, Shams e Tabrizi Street, Iran
Received: May 22, 2018; Accepted: June 08, 2018; Published: June 18, 2018
Abstract
T(14;18)(q32;q21) led to BCL2/IGH fusion, is a common genetic aberration in Follicular Lymphoma (FL) and some high grade B cell lymphomas. This rearrangement can only be envisaged as a primary step in the transformation of a normal B-cell into a malignant cell, more secondary hits are also necessary. Thus t(14;18) translocation might be present in B cells of a percent of healthy individuals, waiting for second hit to get transformed. The aim of this study was to analyze the age-dependent frequency of t(14;18) in the peripheral blood/ lymphoid tissue of a healthy Iranian population to see whether primary or secondary hits were underlying cause of low frequency of FL in Iran. In this study 146 normal samples (peripheral blood/FFPE tissue) examined. Ten positive and 10 negative controls also included. A nested PCR assay was used to investigate BCL2/IGH fusion. Tissue samples with fusion gene detected in them, investigated more deeply by H&E and immunohistochemistry to exclude FL. SPSS version 22 was used for all statistical analysis. Among110 peripheral blood samples, only one showed t(14;18) (0.1%) and two out of 36 FFPE tissue samples were translocation-positive (5.5%). The prevalence of t(14;18)IGH/ BCL2 fusion in Iranian healthy individuals is lower than other populations. It seems that the low frequency of it, as the initial step of carcinogenesis, explains low incidence of FL in Iran. Secondary hits are probably less important in this difference.
Keywords: Follicular lymphoma; Chromosomal translocation t(14;18); IGH/ BCL2 fusion; Healthy individuals
Introduction
It is evident that chromosomal translocations are of prime importance in the genesis of B-cell lymphomas [1]. Non-Hodgkin lymphoma (NHL) is a tumor of B-lymphocyte origin. Follicular lymphoma (FL) accounts for 20-25% of all NHL and is generally characterized by an indolent clinical behavior with an overall median survival of 8 to 10 years. It is recognized as one single entity in the World Health Organization (WHO) classification [2,3]. The t(14;18) (q32;q21) translocation is a common genetic aberration that can be seen as an early step in pathogenesis of FL [4]. It involves the immunoglobulin heavy chain (IgH) gene on chromosome 14q32 and the bcl2 (B cell leukemia/lymphoma 2) gene on chromosome 18q21 and can be detected cytogenetically in about 85-90% of FLs. In other words, the t(14;18)-translocation is not present in all FL cases. Almost 60% of the t(14;18)-translocations are clustered within major breakpoint region (MBR) located in the 3’ untranslated region of the second exon of the bcl2 gene on chromosome 18q21. Another 8-15% of the breakpoints cluster within minor cluster region (mcr) located 25 kb downstream of the MBR [1,5,6]. Nevertheless, breakpoints at bcl- 2 locus are not always located within either the MBR or the mcr, and to their detection need to apply specific methods [7,8]. The breakpoints on chromosome 14q32 are mainly found within the joining elements (JH) of the IgH locus. The location of the breakpoints indicate an aberrant recombination process at a primary stage of pre-B-cell differentiation when the D and J gene elements of the IgH chain locus are rearranged [9]. Of course the origin of these breakpoints is predominantly unknown. In t(14;18)- translocation the anti-apoptotic bcl2 gene comes under the control of the IgH chain enhancer which leads to a constitutive expression of a structural intact, functional bcl2 protein. Therefore, this rearrangement confers a survival advantage to the affected cells by delaying programmed cell death, especially during the follicle center reaction [10-12]. Nonetheless, deregulation of bcl2 alone seems to be insufficient to establish a fully malignant phenotype. This is supported by experiments with transfected cell lines and data obtained from transgenic mice. Therefore, the t(14;18)-translocation can only be envisaged as a primary step in the transformation of a normal B-cell into a malignant cell. Thus, the t(14;18) translocation might be present in blood B cells of healthy individuals [13]. More than 50% of western European and North American normal individuals have circulating B-cells that carry this rearrangement. The frequency of these cells seems to be increased with age and smoking habits measured in pack years. The prevalence in Asian (Japanese) individuals appears to be lower than in Caucasians [14-16]. Follicular lymphoma is uncommon in Iran [17]. The t(14;18)-translocation in cells of normal individuals are indiscernible from those found in FL, and the strongly conserved breakpoints within the bcl2 gene and the igH locus make this aberration a initial target for highly sensitive DNA-PCR techniques [4]. Our aim of this study was to analyze the age-dependent frequency of the t(14;18)- translocation in the peripheral blood/tissue of a healthy Iranian population.
Methods
Study design
This cross-sectional study was done from 2016 to 2017 in the molecular pathology ward of Shiraz Medical University, Shiraz, Iran. The study included 146 healthy samples: One hundred ten peripheral blood samples from live persons and 36 lymphoma tissues from autopsies. Blood and tissue samples of healthy individuals were collected respectively at the affiliated hospital of Shiraz Medical University and forensic medicine, Shiraz, Iran. All live persons and families of deceased persons provided informed consent and the study was approved by the local ethics committee of affiliated hospital of Shiraz Medical University. This study was approved at the Islamic Azad university, Arsanjan, Shiraz. A description of the study healthy population, by gender and age is shown in Table 1.
Variables
live persons
N= 110
Deceased persons
N=36
Gender
Female
Male
53
57
12
24
Mean of age ± SD
91/10 ±53/59
8712 ±86/52
Range of age
40-89
40-85
*Chi-square test.
Table 1: Healthy individuals information.
Also, in this study 10 formalin-fixed, paraffin-embedded (FFPE) tissue samples of FL as positive controls and 5 bone marrow samples of childs that were referred to hospital of shiraz medical university because of other problems not blood disorders as negative controls, have been used. FFPE tissue samples was obtained from archive of pathology ward of shiraz medical university. Blood samples were kept at 4°C until DNA extraction. Autopsy specimens were fixed with 10% formalin and embedded in paraffin blocks.
DNA extraction, nested PCR assay and immunohistochemistry
Genomic DNA was extracted from blood and FFPE samples using the Blood/Cultured cell Genomic DNA Extraction Mini Kit (YTA, cat#YT9040) and Parrafin-fixed Tissue DNA Extraction micro Kit (YTA, cat#:YT9035) respectively, according to the supplier’s protocols. Then, The basic PCR amplification was set as follows: 1 cycle at 94°C for 5 min; 30 cycles at 94°C for 30 s, 55°C for 30 s and 72°C for 40 s; and finally 1 cycle at 72°C for 10 min for extension, to confirm all DNA samples using the actin gene as internal control. The primer sequenses of actin gene are shown in Table 2.
Gene
Sequence (5'→3')
Product size(bp)
Actin
F: CATCTCTTGCTCGAAGTCCA
R: ATCATGTTTGAGACCTTCAA
318
Table 2: Primer sequences of actin gene.
Next, the nested PCR assay was done to investigation of bcl2/ IgH junction. The first PCR reaction of nested PCR contained 2 µL of DNA in a final volume of 25 µL including 12.5 µl of Master mix enzyme (AMPLIQUN,Denmark), 9.5 µl of RNase free double distilled water and 0.5 µl each of outer primer (10 pmol/L).
Then, 0.4 µl of this first 30-cycle PCR product was amplified in a final volume of 25 µL including 12.5 µl of Master mix enzyme (AMPLIQUN,Denmark), 11.1 µl of RNase free double distilled water and 0.5 µµl each of inner primer (10 pmol/L) for the second PCR reaction of nested PCR. PCR conditions were as follows: 1 cycle at 94°C for 5 min for denaturation, 30 cycles at 94°C for 30 sec and 55°C for 30 sec, and finally 1 cycle at 72°C for 10 min for extension. Primers PA1/PA2 is homologous to the 5’ sequence of the MBR and P3/P4 are homologous to the consensus sequence of J1 through J6. The outer and inner primer sequences are summarized in Table 3. The PCR products were analyzed by electrophoresis on a 1.5% agarose gel and by ethidium bromide staining.
Sequence (5'→3')
Product size(bp)
Outer primers
Inner primers
PA1:AGTTATGGCCTATACACTATTTGT
P4:ACCTGAGGAGACGGTGACCAGGGT
PA2:TTGTGAGCAAAGGTGATCGT
P3:CAGGGTCCCTTGGCCCCAG
*
*
*There are different junction fragment sizes [6].
Table 3: The primer sequences used in the nested PCR
As well as, the normal FFPE tissue samples (translocation-positive) were stained immunohistochemically. The primary antibodies used for this study were bcl2, pax5 and ki-67. Therefore, prepared slides of FFPE sections were incubated with primary antibody overnight (18 hours) in a humid chamber followed by secondary antibody incubation for 30 minutes. Finally, DAB chromagen was added. Brown/Red color on slides means the relevant antigen.
Statistical analysis
SPSS version 22 (SSPS, Inc.) was used for all statistical analysis. The categorical data were tested using Pearson’s χ2 test. The significance level was set at P ≤0.05.
Results
Among the 110 healthy individuals whose blood samples were analyzed, 57 were men and 53 were women, and mean of their age was 91/10±53/59 years (range, 40-89 years). Among the 36 deceased whose tissue samples were analyzed, 24 were men and 12 were women, and mean of their age was 8712 ±86/52 years (range, 40-85 years).
Investigation of t(14;18)-translocation in healthy individuals
The frequency of t(14;18)-translocation in healthy individuals are shown in Table 4. Among the 110 samples of peripheral blood, only one sample showed the t(14;18)-translocation and two samples of 36 FFPE tissue samples were translocation-positive. The results of t(14;18)- translocation analysis on agarose gel are shown in Figure1. Based on previous studies, specific band of the t(14;18)- translocation is between 200 to 1 kb. The reason of difference in length of this band, is at igH locus that has 6 subexons. Therefore, it is possible each of the 6 segments be exist in the t(14;18)- translocation[18]. In this study, the length of observed band was between 400 to 450 bp. It is clear in Figure 1 that samples 5,10,11,13 and 14 are translocation-positive. The samples 1-4,6-9 and 15 are negative translocation and sample 16 is negative control. Also, the results of PCR analysis of actin gene to confirm the presence of DNA in the extracted samples, on agarose gel are shown in Figure2.
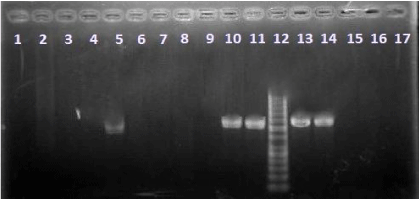
Figure 1: The results of t(14;18)- translocation analysis on agarose gel. Samples 1-4 and 6: Peripheral blood/translocation-negative. Sample 5: Peripheral blood/translocation-positive. Samples 7-9 and 17: FFPE tissues/ translocation-negative. Samples 10 &11: FFPE tissues/translocation-positive. Samples 13&14: positive controls. Samples 15&16: negative controls. Sample 12: ladder (50bp).

Figure 2: The results of PCR analysis of actin gene on agarose gel.
Samples
Translocation-positive
N(%)
Translocation-negative
N(%)
Peripheral blood(N=110)
1(0.91)
109(99.09)
FFPE tissues(N=36)
2(5.55)
34(94.5)
Total
3(2.05)
143(97.95)
Table 4: The frequency of t(14;18)-translocation in healthy individuals.
Investigation of risk factors associated with t(14;18)- translocation
According to information obtained from the participants in the study, The relationship were investigated between risk factors and t(14;18)-translocation. Based on studies, the risk factors are including age, tobacco and etc. The study found no significant association between t(14;18)-translocation and smoking. Also, this translocation did not show a significant correlation with age. The results of this analysis are summarized in Table 5.
Translocation-positive
N(%)
Translocation-negative
N(%)
P-value
People who use tobacco
N=19
0
19(100)
0.999
People who don’t use tobacco
N=91
1(1.1)
90(98.9)
Table 5: The relationship between t(14;18)-translocation and tobacco in blood donors.
Investigation the diagnosis of FL by IHC in normal FFPE tissue samples (translocation-positive)
As mentioned earlier (Table 4), two samples of normal FFPE tissues were translocation-positive. Because one of the exclusion criteria of this study was existence of various diseases specially FL, the samples were analyzed using IHC test. Investigation of samples using IHC different Indicators for FL, were shown in figures 3-6. In Figure 3 and 4, the tissues for the pax5 staining were analyzed with magnification x40 and x200 respectively. It is notable the pax5 staining causes B cell staining. This staining uses for foundation of B cells location in the lymphatic follicles.
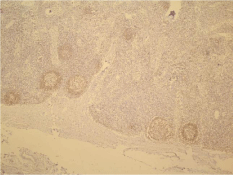
Figure 3: Pax5 staining for the diagnosis of FL with magnification ❌40.
In Figure 4, the larger microscopic view of lymph follicles has been shown that the core of B cells is brown. By this method, B lymphoid follicles and their germinal centers was determined.
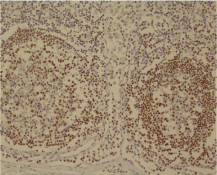
Figure 4: Pax5 staining for the diagnosis of FL with magnification X200.
In Figure 5, the proliferation cells has been shown in the germinal center of one of the lymph follicles. It should be noted that the proliferation cells are brown. As is known, the Power of cell proliferation is high and it is in the interest of follicle hyperplasia. It can not be seen in follicular lymphoma (magnification of x200).
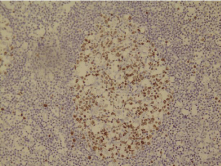
Figure 5: Ki-67 staining for the diagnosis of FL.
In Figure 6, one of the lymph follicles have been shown that its cells colored for the expression of Bcl-2. The germinal center cells do not Bcl-2 expression, while the margin cells are Bcl-2-positive. It is against follicular lymphoma. It should be noted that center cells should be color for Bcl-2 in follicular lymphoma (Figure magnification x200).
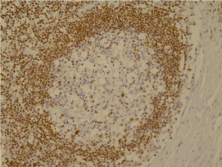
Figure 6: Bcl-2 staining for the diagnosis of FL.
Discussion
Chromosomal translocations involving an oncogene and one of the Ig genes are characteristically found in human B cell lymphomas. Deregulation of the involved oncogene is supposed to be an essential mechanism in the genesis of these tumors. However, similar to solid cancer, malignant lymphomas are likely caused by the occurrence of a series of various oncogenic events. Theoretically, this multistep mechanism might permit us to find Bcells that have accumulated only one or a few but not all hits necessary to establish the malignant phenotype [1]. In the present study, we show that such cells with translocation t(14;18) can be identified in normal individuals. In this study, for the first time the prevalence of t(14;18)-translocation is measured in iran by investigation of lymphatic tissue and the blood. In the current study, the frequency of the t(14;18)- displacement in blood samples and lymph tissues of healthy individuals were 0.91% and 5.5% respectively. Although this study did not show relationship between age and t(14;18), but various studies have noted that the low age can contribute in increasing of the translocation [18]. probably, one of the reasons of this view is the stronger immune system of young people than the elderly. The recombination activating gene (RAG) complex as a nuclease plays an important role in t(14;18)- translocation, and the stronger the immune system can increase the performance of these complex [19-21]. Carsten Hirt, and et al. used epidemiological data and blood samples of the population-based Study of Health in Pomerania (SHIP) to analyze associations of FL risk factors and t(14;18)-positive cells in healthy individuals. Their analyses of t(14;18)-frequency showed a positive association with age but not with sex or smoking [22].The present study also examined the effect of smoking on the incidence of t(14;18)-translocation that significant results was not achieved. It should be noted that the incidence of the t(14;18)-translocation in the western countries is very high. The frequency of this displacement in the western countries has been observed from 50 to 80% [5,23]. For example, in Germany the t(14;18)-translocation can be seen in 52% of normal individuals. Although in another study, which was also conducted in Germany, the t(14;18)- translocation frequency was 46% in healthy individuals [5]. The prevalence of this translocation was mentioned 34% in India [18]. Fuscoe JC, and et al. reported the Prevalence of this displacement 88% (30 of 34) in America [24]. It seems that in communities where the prevalence of follicular lymphoma is high, the prevalence of first hit-t(14;18)-translocation- is also high but in societies like ours the first hit is probably uncommon. Limpens J demonstrated the presence of bcl-2/JH rearrangements in lymph nodes and tonsils with follicular hyperplasia in 13 of 24 cases (54%) [25]. However, in the Asian countries the frequency of this translocation is shown lower (in Japan about 14%) [26]. The present study showed that the frequency of t(14:18)-translocation in the iranian population is much less than the elsewhere in the world. As shown in the results, The frequency of t(14;18)-translocation in the blood and lymphoid tissues is 0.1% and 5.5% respectively. One of the reasons for low frequency of t(14;18)- translocation in iranian healthy individuals is low frequency of FL in this country. Another reason can be the role of secondary factorsin formation of FL. Besides, more frequency of t(14;18)-translocation in lymphoid tissues than blood samples is high frequency of Bcells in lymphoid tissues. As previously mentioned, chromosomal translocations can be used as a marker for hematologic malignancies and, most importantly, leukemia and lymphoma. However the t(14;18)-translocation alone is not sufficient to transform a normal B cell into a malignant lymphoma cell. But such translocation can lead to more cell survival that this advantage occurs with over expression of anti-apoptotic Bcl-2 gene. Then, thereby creating of next hits, the cell may lead to cancerous cell [22]. Therefore, healthy individuals with t(14;18)-translocation can serve as an excellent model to explain lymphomagenesis mechanism.
References
- Limpens J, Stad R, Vos C, de Vlaam C, de Jong D, van Ommen GJ, et al. Lymphoma-associated translocation t (14; 18) in blood B cells of normal individuals. Blood. 1995; 85: 2528-2536.
- Leich E, Salaverria I, Bea S, Zettl A, Wright G, Moreno V, et al. Follicular lymphomas with and without translocation t (14; 18) differ in gene expression profiles and genetic alterations. Blood. 2009; 114: 826-834.
- Godon A, Moreauet A, Talmant P, Baranger-Papot L, Genevie`ve F, Milpied N, et al. Is t(14; 18)(q32; q21) a constant finding in follicular lymphoma? An interphase FISH study on 63 patients. Leukemia. 2003; 17: 255-259
- Schüler F , Dölken L, Hirt C, Kiefer T, Berg T, Fusch G, et al. Prevalence and frequency of circulating t (14; 18)-MBR translocation carrying cells in healthy individuals. Int J Cancer. 2009; 124: 958-963.
- Schüler F, Hirt C, Dölken G. Chromosomal translocation t (14; 18) in healthy individuals. Semin Cancer Biol. 2003; 13: 203-209.
- Liu Y, Hernandezt AM, Shibata D, Cortopassi GA. BCL2 translocation frequency rises with age in humans. Proc Natl Acad Sci U S A. 1994; 91: 8910-8914.
- Weinberg OK, Ma L, Seo K, Beck AH, Pai RK, Morales A, et al. Low Stage Follicular Lymphoma: Biologic and Clinical Characterization According to Nodal or Extranodal Primary Origin. Am J Surg Pathol. 2009; 33: 591–598.
- Albinger-Hegyi A, Hochreutener B, Abdou MT, Hegyi I, Dours-Zimmermann MT, Kurrer MO, et al. High frequency of t (14; 18)-translocation breakpoints outside of major breakpoint and minor cluster regions in follicular lymphomas: improved polymerase chain reaction protocols for their detection. Am J Pathol. 2002; 160: 823-832.
- Bakhshi A, Jensen JP, Goldman P, Wright JJ, McBride OW, Epstein AL, et al. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985; 41: 899-906.
- Dole M, Nunez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, et al. Bcl- 2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994; 54: 3253-3259.
- Vega F, Orduz R, Medeiros LJ. Chromosomal translocations and their role in the pathogenesis of non-Hodgkin’s lymphomas. Pathology. 2002; 34: 397- 409.
- Kirkin V, Joos S, Zörnig M. The role of Bcl-2 family members in tumorigenesis. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2004; 1644: 229-249.
- Summers KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J, et al. Frequency of the Bcl-2/IgH rearrangement in normal individuals: implications for the monitoring of disease in patients with follicular lymphoma. J Clin Oncol. 2001; 19: 420-424.
- Chang CM, Schroeder JC, Huang WY, Dunphy CH, Baric RS, Olshan AF, et al. Non-Hodgkin Lymphoma (NHL) subtypes defined by common translocations: utility of fluorescence in situ hybridization (FISH) in a casecontrol study. Leuk Res. 2010; 34: 190.
- Biagi JJ, Seymour JF. Insights into the molecular pathogenesis of follicular lymphoma arising from analysis of geographic variation. Blood. 2002; 99: 4265-4275.
- Seymour JF. Epidemiology, Pathogenesis and Approaches to Management of Follicular Lymphoma. Korea. 1998; 1466: 57-70.
- Monabati A, Safaei A, Noori S, Mokhtari M, Vahedi A. Subtype distribution of lymphomas in South of Iran, analysis of 1085 cases based on World Health Organization classification. Ann Hematol. 2016; 95: 613-618.
- Nambiar M, Raghavan SC. Prevalence and analysis of t(14;18) and t(11;14) chromosomal translocations in healthy Indian population. Ann Hematol. 2010; 89: 35-43.
- Raghavan SC, Lieber MR. DNA structures at chromosomal translocation sites. Bioessays. 2006; 28: 480-494.
- Raghavan SC, Lieber MR. Chromosomal translocations and non-B DNA structures in the human genome. Cell Cycle. 2004; 3: 760-766.
- Nambiar M, Raghavan SC. Mechanism of fragility at BCL2 gene minor breakpoint cluster region during t(14;18) chromosomal translocation. J Biol Chem. 2012; 287: 8688-8701.
- Hirt C, Weitmann K, Schüler F, Kiefer T, Rabkin CS, Hoffmann W, et al. Circulating t(14;18)-positive cells in healthy individuals: association with age and sex but not with smoking. Leuk Lymphoma. 2013; 54: 2678-2684.
- Rabkin CS, Hirt C, Janz S, Dölken G. t(14;18) Translocations and risk of follicular lymphoma. J Natl Cancer Inst Monogr. 2008; 39: 48-51.
- Fuscoe JC, Woodrow Setzer R, Collard DD, Moore MM. Quantification of t (14; 18) in the lymphocytes of healthy adult humans as a possible biomarker for environmental exposures to carcinogens. Carcinogenesis. 1996; 17: 1013-1020.
- Limpens J, de Jong D, van Krieken JH, Price CG, Young BD, van Ommen GJ, et al. Bcl-2/JH rearrangements in benign lymphoid tissues with follicular hyperplasia. Oncogene. 1991; 6: 2271-2276.
- Yasukawa M, Bando S, Dolken G, Sada E, Yakushijin Y, Fujita S, et al. Low frequency of BCL-2/J H translocation in peripheral blood lymphocytes of healthy Japanese individuals. Blood. 2001; 98: 486-488.