
Special Article – Gynecologic Oncology
Ann Hematol Oncol. 2018; 5(8): 1225.
Predictive Factors for Postrecurrence Survival in Epithelial Ovarian Cancer-a Consecutive Series of 368 Patients and Review of the Literature
Petru E¹*, Idris T¹, Woltsche N², Haas J¹, Benedicic C¹, Heydar-Fadai M¹, Kurschel-Lackner S1,3, Walsberger K¹ and Tamussino K¹
¹Department of Obstetrics and Gynecology, Medical University of Graz, Austria
²Department of Ophthalmology, Medical University of Graz, Austria
³Department of Neurosurgery, Medical University of Graz, Austria
*Corresponding author: Edgar Petru, Department of Obstetrics and Gynecology, Division of Gynecology, Medical University of Graz, Auenbruggerplatz, Austria
Received: November 12, 2018; Accepted: December 27, 2018; Published: December 31, 2018
Abstract
Purpose: Ovarian cancer is usually diagnosed at advanced stage. Thus, recurrences are common. The aim of this retrospective study was to analyze the prognostic value of selected clinical and biological factors with regard to overall survival after first recurrence.
Methods: A total of 368 evaluable patients with primary epithelial cancer of the ovary, fallopian tube or peritoneum were included. Carcinosarcomas, sarcomas and borderline tumors of the ovary, the fallopian tube and the peritoneum were excluded. Patient and tumor characteristics were extracted from hospital records. Recurrence was defined as the first clinical manifestation of tumor progression after an interval of no clinical evidence of disease following primary surgery. Patients with an elevated tumor marker CA125 alone and those who had clinically evident tumor after primary surgery were not eligible for this study
Results: In the multivariate cox regression analysis, five parameters were identified as independent favourable prognostic factors for survival after first recurrence: Time to recurrence = 2 years (p=0.000), Karnofsky status = 80% at the time of recurrence (p=0.008), use of adjuvant chemotherapy (p=0.013), residual disease = 1 cm at primary surgery (p=0.044), and isolated peripheral or paraaortic lymph nodes as localization of first recurrence (p<0.05).
Conclusions: Prolonged interval to recurrence seems to be of utmost importance for longer postrecurrence survival. In addition, small or no residual tumor after primary surgery, administration of adjuvant chemotherapy, higher performance status at recurrence and metastases in the peripheral or paraaortic nodes are predictive for improved overall survival.
Keywords: First recurrence; Ovarian cancer; Prognosis; Survival
Introduction
Most patients with ovarian cancer are diagnosed at an advanced stage, and most will develop recurrence [1]. The abdominal cavity and retroperitoneal lymph nodes are the most frequent sites of first recurrence [1] while distant metastases are uncommon [1,2]. There are inconsistent data on predictive factors for overall survival after first recurrence of epithelial ovarian cancer [3-12].
This retrospective study aimed to describe the patterns of first recurrence and to analyze the predictive value of available clinical and tumor-related factors in patients with ovarian cancer treated and followed up in a single academic institution.
Methods
Between 1976 and 2009, a total of 1,003 consecutive patients with primary epithelial cancer of the ovary, fallopian tube or the peritoneum were treated at the Division of Gynecology, Department of Obstetrics and Gynecology at the Medical University of Graz. Carcinosarcomas, primary sarcomas and borderline were excluded as were tumors metastatic to the ovary. Patient and tumor characteristics were extracted from hospital records and are listed in Table 1.
Characteristics
Number of patients (%)
368 (100%)
Mean age; years (range)
58 (21-90)
Age at primary diagnosis
<70 years
287 (78%)
= 70 years
81 (22%)
Tumor origin
Ovary
344 (93%)
Fallopian tube
14 (4%)
Peritoneum
10 (3%)
FIGO stage
I-II
65 (18%)
III-IV
303 (82%)
Histology
Serous
263 (71%)
Clear-cell
40 (11%)
Endometrioid
32 (9%)
Undifferentiated
21 (6%)
Mucinous
12 (3%)
Grading
G1
42 (11%)
G2
88 (24%)
G3
218 (59%)
Unknown
20 (6%)
Ascites at the time of diagnosis
None
130 (35%)
<500 ml
78 (21%)
>500 ml
160 (44%)
Type of primary surgery
TAHa + BSO/USOb + OM + lymphadenectomyc
184 (50%)
TAHa + BSO/USOb + OMd
184 (50%)
Bowel resection at primary surgery
Yes
110 (30%)
No
256 (69.5%)
Unknown
2 (0.5%)
Retroperitoneal lymph node status
Positive
134 (36%)
Negative
66 (18%)
Unknown
168 (46%)
Residual disease at primary surgery
None
158 (43%)
0.1 to 1.0 cm
45 (12%)
1.1 to 1.9 cm
59 (16%)
> 2.0 cm
106 (29%)
Adjuvant platinum-based chemotherapy
Yes
316 (86%)
No
52 (14%)
Age at first recurrence
<70 years
300 (82%)
= 70 years
57 (15%)
Unknown
11 (3%)
Interval to first recurrence
<12 months
132 (36%)
12-24 months
173 (47%)
>24 months
63 (17%)
Symptoms at recurrence
Yes
229
No
20
Karnofsky status at the time of recurrence/metastasis
< 80
32 (9%)
= 80
336 (91%)
CA125> 40 U/ml at the time of recurrence
<200 U/ml
49 (13%)
=200 U/ml
47 (13%)
Unknown
272 (74%)
Number of sites of recurrence
1
140 (38%)
=2
158 (43%)
>5
70 (19%)
Largest size of recurrent lesion/s
<2 cm
110 (30%)
=2 cm
258 (70%)
Therapy for recurrence
Surgery and platinum-based chemotherapy
14 (4%)
Platinum-based chemotherapy
309 (84%)
Non-platinum-based chemotherapy
26 (7%)
Palliative radiotherapy
2 (1%)
Palliative supportive therapy only
17 (5%)
aTotal abdominal hysterectomy (n=301); previous hysterectomy for benign disease (n=67).
bBilateral salpingo-oophorectomy (n=340), unilateral salpingo-oophorectomy (n=28).
cPelvic (n=184) + paraaortic lymphadenectomy (n=70).
dOmentectomy (n=368).
Table 1: Tumor and patient characteristics of the 368 evaluable consecutive patients with recurrent primary epithelial cancer of the ovary, fallopian tube and peritoneum following primary clinical remission.
Definition of recurrence
Recurrences were identified by retrospective chart review in 389 patients. 21 of these had to be excluded due to incomplete survival data. Thus, a total of 368 patients were analyzed. Recurrence was arbitrarily defined as the first clinical manifestation of tumor progression after clinical remission following primary surgery and chemotherapy. Each recurrence was confirmed by radiologic imaging. In the majority of cases, cytological and/or the clinical course of disease confirmed tumor progression. In the remaining patients, serial imaging and/ or the clinical course of disease unequivocally confirmed tumor progression. Patients with an elevated CA-125 alone and those who had clinically evident tumor after primary surgery were not eligible for this study.
In the vast majority of patients, adjuvant chemotherapy consisted of cisplatinum-based regimens before 1990 and of carboplatin-based regimens thereafter. Carboplatin and paclitaxel combination therapy was used as a standard since the year 2000.
Follow-up visits
Follow-up examinations were scheduled every three months for three years after surgery and every six months until the fifth year after diagnosis. Follow-up had been standardized and carried out centrally at our institution for 95% of patients. Follow-up consisted of gynecologic clinical examinations every three months during the first three years as well as six-monthly CT scans thereafter until the end of the fifth year after diagnosis.
Predictive factors analyzed
The following factors potentially affecting postrecurrence survival were analyzed:
- Primary tumor origin
- FIGO stage
- Histology
- Grading
- Age at primary diagnosis
- Ascites at the time of diagnosis
- Type of primary surgery
- Bowel resection at primary surgery
- Retroperitoneal lymph node status
- Residual disease at primary surgery
- Adjuvant platinum-based chemotherapy
- Interval to first recurrence
- Symptoms at recurrence
- Karnofsky status at the time of recurrence/metastasis
- CA125 > 40 U/ml at the time of recurrence
- Localization of recurrence
- Number of sites of recurrence
- Largest size of recurrent lesions
- Therapy for recurrence
Statistical analysis
Standard descriptives methods were used. Kaplan-Meier-curves were applied to describe patient survival from the time of recurrence, and Cox’s proportional hazards model was used for multivariate analysis.
The analysis was done with IBM SPSS (IBM SPSS Statistics for Windows, V.22. Armonk, NY: IBM Corp).
Results
The mean follow-up of study patients was 17 months (range 0 to 146 months). The median post-recurrence survival was 11 months (range 0-146 months). Recurrences in the 368 patients were diagnosed after a median of 16 months (range 0-186 months). Sites of recurrence are shown in (Table 2).
Characteristics
Number of patients (%)
368 (100%)
Peritoneum
90 (25%)
Peritoneum and pelvis
14 (4%)
Pelvis only (no specification)
87 (24%)
Pelvic side wall
11 (3%)
Vaginal cuff
15 (4%)
Middle and lower vagina
4 (1%)
Liver
22 (6%)
Liver and peritoneum
15 (4%)
Liver and pelvis
4 (1%)
Liver and pleura
2 (0.5%)
Pleura
8 (2%)
Pleura and peritoneum
4 (1%)
Pleura, peritoneum and pelvis
3 (1%)
Pleura and lung
2 (0.5%)
Lung
11 (3%)
Paraaortic lymph nodes only
23 (6%)
Paraaortic lymph nodes and liver
4 (1%)
Paraaortic and mediastinal lymph nodes
1 (0.3%)
Paraaortic lymph nodes and peritoneum
1 (0.3%)
Paraaortic lymph nodes and pelvis
1 (0.3%)
Paraaortic lymph nodes, peritoneum and pelvis
1 (0.3%)
Paraaortic lymph nodes and pleura
1 (0.3%)
Supraclavicular lymph nodes
9 (2%)
Supraclavicular lymph nodes and pelvis
1 (0.3%)
Inguinal lymph nodes
7 (2%)
Inguinal lymph nodes and peritoneum
1 (0.3%)
Inguinal and axillary lymph nodes,
peritoneum and lung
1 (0.3%)
Brain
8 (2%)
Spleen
4 (1%)
Spleen and peritoneum
3 (1%)
Skin
4 (1%)
Umbilicus
3 (1%)
Abdominal wall
3 (1%)
Table 2: Localization of first recurrence/metastasis in the 368 evaluable consecutive patients with primary epithelial cancer of the ovary, fallopian tube and peritoneum.
Univariate analysis identified the following parameters to be significantly associated with favourable survival after first recurrence: FIGO stage I-II (p=0.000), tumor residuals of = 1cm in diameter at primary surgery (p=0.000), only one localization of recurrence (p=0.000), age < 70 years at the time of recurrence (p=0.017), negative retroperitoneal lymph node status at primary surgery (p=0.018), and peripheral lymph node recurrence (p=0.009), respectively.
In multivariate Cox regression analysis, five parameters were identified as independent favorable predictive factors for survival after first recurrence: Time to recurrence = 2 years (p=0.000; Figure 1), Karnofsky status = 80% at the time of recurrence (p=0.008, Figure 2), use of adjuvant platinum-based chemotherapy (p=0.013), residual disease = 1cm at primary surgery (p=0.044, Figure 3) and isolated peripheral or paraaortic lymph nodes as localization of first recurrence (p<0.05; Figure 4), respectively.
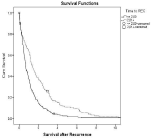
Figure 1: Overall cumulative survival of patients with primary epithelial
cancer of the ovary, fallopian tube, or peritoneum whose interval between
primary diagnosis and recurrence was less than or equal two years versus
those with an interval of greater than two years.
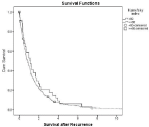
Figure 2: Overall cumulative survival of patients with primary epithelial
cancer of the ovary, fallopian tube, or peritoneum who either had a Karnofsky
status of < 80% or = 80% at the time of recurrence.
Discussion
In 368 patients who developed recurrence after clinical remission, postrecurrence survival was favorably influenced by an interval of = 2 years from primary surgery (Figure 1), small or no residual disease at primary surgery (Figure 3), the use of adjuvant platinum-based chemotherapy, a higher Karnofsky status at recurrence (Figure 2) and recurrence/metastases in the peripheral or paraaortic lymph nodes (Figure 4).
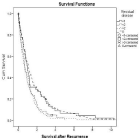
Figure 3: Overall cumulative survival of patients with primary epithelial
cancer of the ovary, fallopian tube, or peritoneum who either had no residual
disease, residual disease < 1 cm, residual disease between 1.0 and 1.9 cm,
or = 2 cm in largest diameter at primary debulking surgery.
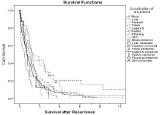
Figure 4: Overall cumulative survival of patients with primary epithelial
cancer of the ovary, fallopian tube, or peritoneum who either developed their
first recurrence in the brain, parenchymatous liver, paraaortic lymph nodes,
pelvis, peripheral lymph nodes, peritoneum, pleura and lungs, and skin,
respectively.
There are only three studies including higher patient numbers with ovarian cancer recurrence [9,10,13]. However, one of these had focussed on the response of patients to chemotherapy only [13].
Longer interval to recurrence
Our study identified a disease-free interval of two years from diagnosis as predictor of improved survival (Figure 1; p=0.000). This finding supports several other reports [3-8,10,11].
Residual disease = 1cm at primary surgery
In the present study, minimal or no residual disease at primary surgery was an independent favorable factor for postrecurrence survival (Figure 3; p=0.044). This finding is supported by another report on pooled data from 3 randomized AGO studies from the German Working Group for Gynecologic Oncology [9].
Adjuvant chemotherapy
The administration of adjuvant platinum-based chemotherapy was identified as favourable predictive factor for prognosis in the present series (p=0.013). We were unable to identify other studies showing a similar finding.
Higher performance status at the time of recurrence
Our findings revealed a Karnofsky status = 80% at the time of recurrence to be predictive for postrecurrence survival (p=0.008; Figure 2). Other groups have shown similar results [3,6].
Localization of recurrence
The most common first clinical manifestations of recurrent ovarian cancer were metastases of the peritoneal cavity, followed by the pelvis. Isolated lymph node recurrences, mainly concerning the paraaortic area, were less common (Table 2).
Peritoneal metastases
It seems evident that the term peritoneal cavity was not uniformly used in the studies reported since some authors have not distinguished between recurrences restricted to the pelvis and those involving both the pelvis and the peritoneal cavity [1,14-16]. The present study did not identify a prognostic difference between these two recurrence patterns (Figure 4).
Peritoneal metastases have been identified as a component of first recurrence in 36% of our patients (Table 2). This data are in line with those from another study (38% peritoneal recurrences; [1]). Amate et al. reported peritoneal involvement as site of first recurrence in 77% of patients, most of them having advanced disease [16].
In autopsy studies, up to 96% of patients were identified to have peritoneal metastatic involvement [14,15,17].
Pelvic metastases
In our cohort, 34% of patients showed first recurrences in the pelvis (Table 2). In contrast, an autopsy study revealed even a higher rate of pelvic peritoneal metastases (65%; [17]).
Isolated vaginal cuff recurrences
In our collective, 15 patients (4%) developed an isolated recurrence of the vaginal cuff (Table 2). This is in accordance with another study in which 5% such recurrences were reported [18].
Liver metastases
Parenchymatous liver metastases occurred in 13% of our patients (Table 2). Dauplat et al. reported 14% liver metastases [19]. Autopsy studies in patients with ovarian cancer have observed liver metastases between 45 and 52% of patients [14,15,17].
Peripheral and paraaortic lymph node recurrences
In the present series, metastatic involvement of peripheral or paraaortic lymph nodes was found in 51 cases 39 of which (77%) were isolated (Table 2). Overall, peripheral and paraaortic lymph node recurrences represented 11% of recurrences in this series. Paraaortic recurrences were treated by secondary surgery in 10 patients. Peripheral or paraaortic lymph node involvement was a favorable predictor of survival in the present study (Figure 4; p<0.05). This data is in line with several previous reports in which survival up to > 100 months was recorded [11,20-22].
Pleural metastases
About 5% of our patients had pleural metastases as primary localization of recurrence (Table 2). In studies with far advanced disease and autopsy studies, the proportion of pleural involvement lay between 28 and 59%, respectively [14,15,17]. In a report on 97 patients first recurring at distant sites, 59% had malignant pleural effusions [19]. Another study showed thoracic involvement in 45% of patients the vast majority of them having pleural effusions [23].
Lung metastases
In the present study, parenchymal lung metastases were found as primary manifestation of recurrence in 4% of patients only (Table 2). This number corresponds well to one other study [23]. Autopsy studies have found a prevalence of lung metastases in up to 39% [14,15,17].
Spleen metastases
Two percent of patients in the present series showed metastatic involvement of the spleen (Table 2). Previous studies have revealed rates between 4% and 10%, respectively [1,2]. However, autopsy studies have reported spleen metastases in 19% to 51% of patients, respectively [14,15,17].
Brain/CNS metastases
In the present cohort, 2% of patients were diagnosed with brain metastases (Table 2). These data are in line with previous observations by Dauplat et al [19]. Autopsy studies have revealed up to 6% brain metastases from ovarian cancer [14,15,17].
Skin metastases
In the present series, four patients (1%) developed skin metastases (Table 2). These data are in line with Cormio et al. who described 4% skin metastases among 220 patients with epithelial ovarian cancer [2]. Autopsy studies revealed prevalence rates of as high as 5% [15,17].
Number of sites of recurrence
The number of sites of recurrence was not predictive for postrecurrence survival in the present series. However, various and inconsistent methods have been applied in order to diagnose the localization of recurrences/metastases. For example, a patient might have undergone emergency surgery for an ileus at the time of first recurrence at which numerous metastases were stated. In another case, a patient with significant dyspnea might have been diagnosed via chest X-ray only without performing further imaging. In the present study, this patient might have been classified as having one localization of recurrence only despite the coexistence of several other metastatic lesions identified 2 weeks later by thoracoscopy.
In one study, the number of disease sites at recurrence was significantly associated with response. However, the authors did not report on overall survival [13]. Biliatis et al. reported that three or more sites of recurrence are independently associated with poor prognosis [7]. Pignata et al. reported similar effects on overall survival [6].
Maximum tumor size at recurrence
This parameter was not significantly associated with survival in the present dataset. One pooled study found tumor size of recurrence = 5 cm to be independently associated with improved response [13].
FIGO stage
In the present cohort, 303 patients (82%) had FIGO stage III or IV disease. Stage had no influence on postrecurrence survival. In contrast, two other studies have identified advanced stage of disease as a negative independent prognostic factor post recurrence [7,10].
Histological subtype
The histological subtype had no predictive value in the present series. Only singular studies have found serous histology to be significantly associated with an improved response and prognosis [7,10,13].
CA 125
In the present study, CA-125 values were available only in 26% of patients overall (Table 1). No predictive value was found. Another group also reported no prognostic significance for preoperative CA 125 evaluated in patients before secondary cytoreduction [5].
Other factors investigated
No difference in postrecurrence survival was found with regard to the origin of the primary tumor, grading, age at diagnosis and recurrence, the presence of ascites at primary surgery, whether primary surgery included lymphadenectomy or not, retroperitoneal lymph node status, and type of treatment of recurrence. However, it has to be stated that only a minority of our patients underwent secondary debulking surgery (4%; Table 1). There are several papers in the literature which have shown secondary debulking surgery at the time of recurrence to positively influence survival in selected patients if residual disease is low [24].
Conclusion
In this series, the time to recurrence was the strongest predictor of postrecurrence survival. In addition, small or no residual tumor after primary surgery, the administration of adjuvant chemotherapy, a higher performance status at recurrence, and metastases in the peripheral or paraaortic nodes are predictive for overall survival. Thus, even parameters relevant at the time of diagnosis and shortly thereafter, such as residual tumor size and/or adjuvant chemotherapy influence postrecurrence survival of patients with recurrent ovarian cancer.
Author Contributions
All authors have contributed intellectually to the manuscript.
EP: Study concept and design, acquisition of clinical data, the use of manuscript writing.
TI: Acquisition of clinical data, manuscript writing.
NW: Acquisition of clinical data, manuscript writing.
JH: Statistical analysis, interpretation of data.
CB: Acquisition of clinical data, manuscript writing.
MH: Acquisition of clinical data, manuscript writing.
SK: Acquisition of clinical data, manuscript writing.
KW: Acquisition of clinical data, manuscript writing.
KT: Manuscript editing.
References
- Kikkawa F, Kawai M, Mizuno K, Ishikawa H, Kojima M, Maeda O, et al. Recurrence of epithelial ovarian carcinoma after clinical remission. Gynecol Obstet Invest. 1994; 38: 65-69.
- Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003; 13: 125-129.
- Begum F, Høgdall E, Riisbro R, Christensen I, Engelholm S, Jørgensen M, et al. Prognostic value of plasma soluble urokinase Plasminogen Activator Receptor (suPAR) in Danish patients with Recurrent Epithelial Ovarian Cancer (REOC). APMIS. 2006; 114: 675-681.
- Chan JK, Tian C, Teoh D, Monk BJ, Herzog T, Kapp DS, et al. Survival after recurrence in early-stage high-risk epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2010; 116: 307-311.
- Mahner S, Woelber L, Jung S, Eulenburg C, Ihnen M, Schwarz J, et al. Prognostic significance of CA-125 in the management of patients with recurrent epithelial ovarian carcinoma selected for secondary cytoreduction. Anticancer Res. 2009; 29: 2817-2821.
- Pignata S, Ferrandina G, Scarfone G, Scollo P, Odicino F, Cormio G, et al. Poor outcome of elderly patients with platinum-sensitive recurrent ovarian cancer: results from the SOCRATES retrospective study. Crit Rev Oncol Hematol. 2009; 71: 233-241.
- Biliatis I, Haidopoulos D, Rodolakis A, Vlachos G, Protopapas A, Thomakos N, et al. Survival after secondary cytoreduction for recurrent ovarian cancer: which are the prognostic factors?. J Surg Oncol. 2010; 102: 671-675.
- Classe J, Jaffre I, Frenel J, Bordes V, Dejode M, Dravet F, et al. Prognostic factors for patients treated for a recurrent FIGO stage III ovarian cancer: a retrospective study of 108 cases. Eur J Surg Oncol. 2011; 37: 971-977.
- Hanker L, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, et al. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012; 23: 2605-2612.
- Kajiyama H, Shibata K, Mizuno M, Umezu T, Suzuki S, Yamamoto E, et al. Long-term clinical outcome of patients with recurrent epithelial ovarian carcinoma: is it the same for each histological type?. Int J Gynecol Cancer. 2012; 22: 394-399.
- Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014; 384: 1376-1388.
- Gadducci A, Cosio S, Zola P, Sostegni B, Fuso L, Sartori E. Prognostic factors and clinical outcome of patients with recurrent early-stage epithelial ovarian cancer: an Italian multicenter retrospective study. Int J Gynecol Cancer. 2013; 23: 461-468.
- Eisenhauer E, Vermorken J, van Glabbeke M. Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients. Ann Oncol. 1997; 8: 963-968.
- Abrams H, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950; 3: 74-85.
- Rose P, Piver M, Tsukada Y, Lau T. Metastatic patterns in histologic variants of ovarian cancer. An autopsy study. Cancer. 1989; 64: 1508-1513.
- Amate P, Huchon C, Dessapt A, Bensaid C, Medioni J, Le Frère Belda M, et al. Ovarian cancer: sites of recurrence. Int J Gynecol Cancer. 2013; 23: 1590-1596.
- Dvoretsky P, Richards K, Angel C, Rabinowitz L, Stoler M, Beecham J, et al. Distribution of disease at autopsy in 100 women with ovarian cancer. Hum Pathol. 1988; 19: 57-63.
- Casey A, Park M, Holschneider C, Bozuk M, Punyasavatsut M, Montz F. Apical vaginal recurrence of ovarian carcinoma: presentation, treatment and survival. Int J Gynecol Cancer. 1996; 6: 200-204.
- Dauplat J, Hacker N, Nieberg R, Berek J, Rose T, Sagae S. Distant metastases in epithelial ovarian carcinoma. Cancer. 1987; 60: 1561-1566.
- Uzan C, Morice P, Rey A, Pautier P, Camatte S, Lhomme C, et al. Outcomes after combined therapy including surgical resection in patients with epithelial ovarian cancer recurrence(s) exclusively in lymph nodes. Int J Gynecol Cancer (Abstracts). 2004; 14: 42-43.
- Blanchard P, Plantade A, Pagès C, Afchain P, Louvet C, Tournigand C, et al. Isolated lymph node relapse of epithelial ovarian carcinoma: outcomes and prognostic factors. Gynecol Oncol. 2007; 104: 41-45.
- Legge F, Petrillo M, Adamo V, Pisconti S, Scambia G, Ferrandina G. Epithelial ovarian cancer relapsing as isolated lymph node disease: natural history and clinical outcome. BMC Cancer. 2008; 8: 367.
- Kerr V, Cadman E. Pulmonary metastases in ovarian cancer. Cancer. 1985. 56: 1209-1213.
- Harter P, Sehouli J, Reuss A, Hasenburg A, Scambia G, Cibula D, et al. Prospective validation study of a predictive score for operability of recurrent ovarian cancer: the Multicenter Intergroup Study DESKTOP II. A project of the AGO Kommission OVAR, AGO Study Group, NOGGO, AGO-Austria, and MITO. Int J Gynecol Cancer. 2011; 21: 289-295.