
Case Presentation
Ann Hematol Oncol. 2019; 6(1): 1226.
Azacitidine Switch to Lenalidomide Eradicated the TP53/ CDKN2A Co-Mutated Clone and Induced Long-Term Erythroid Response in Del(5q) MDS
Minarik L1,2, Zemanova Z3, Kulvait V1, Dluhosova M1, Jonasova A2* and Stopka T1,2*
1Biocev, First Medical Faculty, Charles University, Vestec, Czech Republic
2First Internal Clinic-Clinic of Hematology, General University Hospital, Prague, Czech Republic
3Center of Oncocytogenetics, General University Hospital, Prague, Czech Republic
*Corresponding author: Stopka T & Jonasova A, First Medical Faculty and General Hospital, Charles University, Prague, Czech Republic
Received: November 08, 2018; Accepted: January 07, 2019;Published: January 14, 2019
Abstract
Management of progressing Myelodysplastic Syndrome (MDS) represents a very difficult task for clinicians. While targeted therapy with lenalidomide is administered at low-risk phase and is primarily effective in patients with deletion of chromosome 5q, it is largely ineffective upon progression at high-risk MDS phase characterized by accumulation of adverse somatic mutations involving the tumor suppressor protein TP53. We herein report a 68-year old male MDS del(5q) patient that progressed to higher risk MDS EB1 with red blood cell transfusion dependency. Administration of 17 cycles of azacitidine inhibited further progression by stabilizing disease with Complete Marrow Response (mCR) without hematology improvement. The patient despite of reaching mCR on AZA further increased the allelic burden of gene mutations in the Cyclin- Dependent Kinase Inhibitor 2A, CDKN2A at residue D74A, Tumor Suppressor TP53 (K373R+T377P), and the splicing factor ZRSR2 (P13T). Based on the fact that the patient reached lower blast count on AZA but was accumulating adverse mutations we decided to switch the therapy into lenalidomide. The lenalidomide therapy repelled the progression-prone subclones characterized by the somatic mutations, fully normalized blood counts, and produced a longlasting remission. Our data suggest that the del(5q) patient progressing to high risk MDS could be treated by azacitidine to block MDS progression, however, only additional therapeutic line of lenalidomide was capable to suppress the progression-prone clones characterized by unfavorable mutations involving also TP53.
Keywords: Myelodysplastic syndrome; Azacitidine; Lenalidomide; NGS; ddPCR
Abbreviations
EPO: Erythropoietin; AZA: Azacitidine; LEN: Lenalidomide; TU: Transfusion Unit; HB: Hemoglobin; MCV: Mean Corpuscular Volume; DG: Diagnosis; MDS: Myelodysplastic Syndrome
Case Presentation
Management of progressing Myelodysplastic Syndrome (MDS) represents a very difficult task for clinicians. While targeted therapy with Lenalidomide (LEN) is administered at low-risk phase and is primarily effective in patients with deletion of chromosome 5q, it is largely ineffective upon progression at High-Risk (HR) MDS phase characterized by accumulation of adverse somatic mutations involving the tumor suppressor protein TP53. LEN therapy is not administered to MDS-progressing patients. However, it may be of clinical benefit if LEN could prolong the Azacitidine (AZA)-mediated response that is often complicated by AZA-resistance. We herein present longitudinal data from MDS del(5q) patient that progressed to HR-MDS EB1 with red blood cell transfusion dependency. While administration AZA induced the Complete marrow Response (mCR) the switch to LEN therapy repelled the progression-prone sub clones characterized by adverse somatic mutations and restored hematology parameters.
MDS progression is often associated with the accumulation of genetic aberrations that allow high survival properties for myeloblast outgrowth. In MDS subtype characterized by chromosome 5q deletion, the sensitivity to LEN treatment may lead to red blood cell recovery of transfusion-dependent anemia. LEN therapy induces tumor-specific cell lethality via Cereblon-dependent degradation of haplodeficient proteins encoded within the commonly deleted region at chromosome 5q. In non-del(5q) MDS, LEN sensitivity was documented to enhance the Erythropoietin (EPO) receptor-initiated transcriptional response. Monitoring of cytogenetic aberrations by FISH and nucleotide variants by NGS provides useful data for assessment of clinical outcomes example includes a study showing that non-del(5q) ancestral clones containing a distinct pattern of mutations may expanded over time on LEN [1]. Inversely, loss of LEN sensitivity may lead to re-expansion of del(5q) clone with transient sensitivity to DNA-methylation inhibitor AZA [1]. Despite the promising potential of AZA and LEN therapy to block progression in HR-MDS and to induce complete remission, the AZA-based combinations had similar response rate (ORR) to AZA alone (Sekeres, Othus et al. 2017). The del(5q) MDS patients may progress to HR-MDS, which is indicated for AZA therapy. Since this process has been in some patients associated with mutations of tumor suppressor TP53, the response to AZA may be limited. Allele burden of the TP53 mutant clone in del(5q) MDS is inversely proportional to Overall Survival (OS), confirming the importance of p53 as a negative prognostic variable of therapy response [2]. However, recently we and also others have observed that some but not all MDS patients with TP53 mutations treated by AZA have relatively longer survival, which creates a possibility that AZA can partly control growth of these clones [3]. To ensure that the mutation-bearing clone was a target of therapy it is essential to monitor the mutation pattern in respect to therapeutic lines.
We herein present complex clinical, laboratory and molecular data of 68-year-old male MDS patient that was initially diagnosed with MDS with isolated cytogenetically detected del(5q) in 46% of cells. Risk of progression was calculated as low (IPSS-R was determined to be 3=intermediate). However, after 14 months the patient became transfusion-dependent as he progressed to EB1 (excess of blasts 1). 4-months EPO administration achieved no response. Besides cytogenetic del(5q) aberration (detected by standard and advanced FISH technology), his Bone Marrow (BM) contained 7% Myeloblasts (MB). BM was analyzed by TruSight Myeloid Sequencing Panel (Illumina, San Diego, USA) that determines integrity of 54 target gene regions and is a set of 568 amplicons and ~141 kb designed to detect somatic variants previously associated with myeloid malignancies.
Mutations of CDKN2A (D74A) and TP53 (K373R) with pathogenic FATHMM score 0.71 were noted in BM CD3-depleted myeloid cells. The TP53 K373R mutation has been previously described in AML [4] and reported in the COSMIC (https://cancer.sanger.ac.uk/cosmic) database. Additional two less-pathogenic variants in TP53 (T377P) and ZRSR2 (P13T) also exceeded a 5% cutoff. Next, the patient was treated with AZA 75mg/m2 (5-2-2 regimen). After 4 cycles and 13 cycles the restaging analyses indicated the marrow CR. Nevertheless, the transfusion dependency (>4 transfusion units/6 weeks) stayed unimproved with AZA as the patient collectively received 72 blood units, which aggravated his iron overload characterized by increasing Ferritin levels. Importantly, tumor burden measured as a function of abundance of del(5q) + the somatic mutations was not affected by AZA (Figure 1).
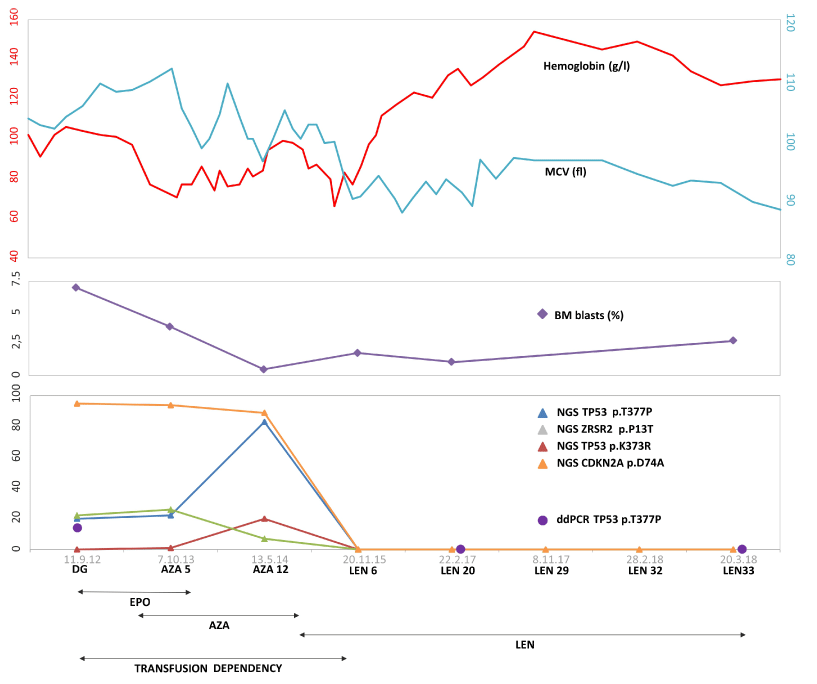
Figure 1: Timeline of patients’ disease course. Shortly after diagnosis of del(5q) MDS there was a development of transfusion dependency, patient started the EPO
therapy, however without any effect. Control BM examinations indicated progression to EB1 and the patient started the AZA treatment lasting for 17 cycles, which
led to a decrease of bone marrow blasts (mCR) but concurrently increased the allelic burden of somatic mutations. The patient remained transfusion-dependent and
therefore the therapy was switched to LEN that led to a complete response (Hemoglobin levels and Mean corpuscular volumes are shown at the top). Middle panel
shows dynamics of bone marrow blast counts and variant allele frequencies (in %) of somatic mutations during the therapies. Besides persisting del(5q) aberration
the patient contained also mutations in CDKN2A D74A, TP53 K373R+T377P and ZRSR2 P13T; the clone/s was eradicated by LEN therapy.
The patient, being scored as mCR without Hematology Improvement (HI) was after 14 months on AZA switched to LEN. Shortly after 5 weeks of LEN he became transfusion-independent with normal Blood Counts (CBC). Repeated bone marrow examinations showed less than 5% MB and patient stayed transfusion-independent. Cytogenetic analyses after 6 and 20 cycles of LEN indicated stable abundance of del(5q). NGS analyses at these restaging time points however indicated that all four somatic mutations detected in earlier time points that were resistant to AZA (CDKN2A D74A, TP53 K373R+T377P, and ZRSR2) became absent following LEN therapy. The NGS data were confirmed using digital PCR technology for the TP53 T377P variant in time points after 20 and 33 cycles of LEN in both peripheral blood and BM and indicated that the progressionprone clone was undetectable even with this highly sensitive technology. Together, the del(5q) HR-MDS patient treated with AZA (14 months) as EB1 achieved mCR. Upon switch to LEN therapy the patient gained HI and transfusion independency with remaining signs of BM dysplasia, however concomitantly to the clearance of clones bearing pathogenic mutations.
Discussion/Conclusion
Herein presented data are backed by well-accepted notion that LEN can induce erythropoietic response and transfusionindependence in 60-70% of MDS del(5q) patients, but only 20% achieve complete cytogenetic response [5]. Our data document that the progression-prone clone/s characterized by CDKN2A D74A, TP53 K373R+T377P and ZRSR2 P13T could be controlled by LEN, which precludes concomitant presence of del(5q) aberration within the same sub clone/s and agreeing with a study that suggested importance of TP53 mutations for progression in low-risk MDS patients with del(5q) [6]. However, a recent study implicated that cumulative incidence of progression was similar when TP53 mutations were detected (or not) at diagnosis, while in patients with clonally evolving the TP53 mutation/s the cumulative incidence of progression was tremendously high [7]. Others indicated that a TP53 mutation rather precludes lower sensitivity to LEN treatment [2]. It is possible that treatment with AZA prior to LEN could have selected additional subliminal clones that were sensitive to LEN therapy. On the contrary, LEN-resistant and progressing MDS patients can benefit from AZA effect on improving patient survival [8]. Our other work indicated that del(5q) aberration appears to be the most important positive predictor of longer overall survival and response duration on AZA in higher risk MDS patients [3]. Collectively, we present a clinical experience of MDS del5q progression that can be effectively managed partially by AZA until the progression is blocked, followed by a switch to LEN therapy, which proved to be very efficient with long-lasting clinical effects that could be explained by its ability to repel progression-prone MDS subclones carrying adverse gene mutations.
Ethics Approval and Consent to Participate
This study was approved by the Institutional ethical review board of General University Hospital, Prague.
Consent for Publication
Informed consent from patient on collecting anonymized information and patient’s samples was obtained.
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ Contributions
LM: Genetic Analyses, ZZ: Cytogenetics, AJ: Clinical Work, MD: Laboratory Analyses, TS: Research Design & Writing.
Acknowledgement
Authors acknowledge help of Dr. Katerina Hodanova with NGS run at the National Center for Medical Genomics (CZ.02.1.01/0.0/0. 0/16_013/0001634).
Funding
GACR 16-05649S, 18-01687S, AZV 16-27790A, UNCE/MED/016, SVV 260374/2017, Progres-Q26, LQ1604, CZ.1.05/1.1.00/02.0109, CZ.01.1.02/0.0/0.0/16_083/0010326 and GAUK 528513 (TS,AJ,LM, MD), RVO-VFN64165 and P302/12/G157 (ZZ).
References
- Da Silva-Coelho P, Kroeze LI, Yoshida K, Koorenhof-Scheele TN, Knops R, van de Locht LT, et al. Clonal evolution in myelodysplastic syndromes. Nat Commun. 2017; 8: 15099.
- Mossner M, Jann JC, Nowak D, Platzbecker U, Giagounidis A, Gotze K, et al. Prevalence, clonal dynamics and clinical impact of TP53 mutations in patients with myelodysplastic syndrome with isolated deletion (5q) treated with lenalidomide: results from a prospective multicenter study of the german MDS study group (GMDS). Leukemia. 2016; 30: 1956-1959.
- Polgarova K, Vargova K, Kulvait V, Dusilkova N, Minarik L, Zemanova Z, et al. Somatic mutation dynamics in MDS patients treated with azacitidine indicate clonal selection in patients-responders. Oncotarget. 2017; 8: 111966-11978.
- Conte N, Varela I, Grove C, Manes N, Yusa K, Moreno T, et al. Detailed molecular characterisation of acute myeloid leukaemia with a normal karyotype using targeted DNA capture. Leukemia. 2013; 27: 1820-1825.
- Fenaux P, Giagounidis A, Selleslag D, Beyne-Rauzy O, Mufti G, Mittelman M, et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood. 2011; 118: 3765-3776.
- Jadersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Gohring G, et al. TP53 mutations in low-risk myelodysplastic syndromes with del(5q) predict disease progression. J Clin Oncol. 2011; 29: 1971-1979.
- Lode L, Menard A, Flet L, Richebourg S, Loirat M, Eveillard M, et al. Emergence and evolution of TP53 mutations are a key feature of disease progression in myelodysplastic patients with lower-risk del(5q) treated with lenalidomide. Haematologica. 2018; 103: e143-e146.
- Prebet T, Cluzeau T, Park S, Sekeres MA, Germing U, Ades L, et al. Outcome of patients treated for myelodysplastic syndromes with 5q deletion after failure of lenalidomide therapy. Oncotarget. 2017; 8: 81926-81935.