
Research Article
Ann Hematol Oncol. 2019; 6(5): 1248.
A Risk-Scoring System to Predict Heart Failure Onset during Treatment of Myelodysplastic Syndrome with Azacitidine
Kambara Y1,2, Yamamoto A1, Masunari T1, Sezaki N1, Sugiura H1,2, Ikegawa S2, Meguri Y1,2* Kiguchi T1* and Maeda Y2
1Department of Hematology, Chugoku Central Hospital, Hiroshima, Japan
2Department of Hematology and Oncology, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama, Japan
*Corresponding author: Meguri Y, Department of Hematology and Oncology, Okayama University Graduate School of Medicine Dentistry and Pharmaceutical Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama City, Okayama 700-8558, Japan
Kiguchi T, Department of Hematology, Chugoku Central Hospital, 148-13 Fukuyama City, Miyuki-Cho Kamiiwanari, Hiroshima 720-0001, Japan
Received: March 09, 2019; Accepted: April 04, 2019;Published: April 11, 2019
Abstract
Cardio-oncology is increasingly being recognized as an important field of medicine. Surveillance of cardiac events is important for patients treated with agents that can improve survival, such as azacitidine, a hypomethylating agent for treating Myelodysplastic Syndrome (MDS). Thus, we aimed to develop a riskscoring system to predict Heart Failure (HF) onset during azacitidine therapy and to investigate factors associated with HF onset and causes of death.
Sixty patients with MDS or acute myeloid leukemia who were treated with azacitidine were included. Diagnosis of HF was made using Framingham Heart Study Criteria. We used Gray test for univariate analysis and fine-Gray test for multivariate analysis to confirm the cumulative incidence of risk factors.
HF occurred in 15 (25%) patients. Univariate and multivariate analyses showed that the history of heart disease (Odds Ratio [OR], 4.78; 95% Confidence Interval [CI], 1.51-15.2), red blood cell transfusion dependency (OR, 5.21; 95% CI, 1.59-17.0), and >50000 copies/μg RNA of Wilms’ tumor gene-1 mRNA (OR, 3.49; 95% CI, 1.15-10.5) were independent risk factors of HF onset. By using these risk factors, we developed a scoring system to predict HF onset, which stratified patients into three groups. There was a significant difference in cumulative incidence (2-year cumulative incidence; 95% CI; low risk, 0.00%, 0.00-0.00; intermediate risk, 21.4%, 8.50-38.2; high risk, 65.9%, 28.3-87.1; p ‹0.01).
This scoring system is effective for the management of patients with MDS. This is the first report of an HF risk-scoring system for predicting HF onset during azacitidine therapy.
Keywords: Acute myeloid leukemia; Azacitidine; Cardio-oncology; Heart failure; Myelodysplastic syndrome
Abbreviations
MDS: Myelodysplastic Syndrome; HF: Heart Failure; AML: Acute Myeloid Leukemia; CHF: Congestive HF; RBC: Red Blood Cell; OR: Odds Ratio; CI: Confidence Interval; BNP: Brain Natriuretic Peptide; EF: Ejection Fraction: WT-1: Wilms’ Tumor Gene-1; OMI: Old Myocardial Infarction; CKD: Chronic Kidney Disease; CRP: C-Reactive Protein
Introduction
Cardio-oncology is increasingly being recognized as an important field of medicine. Cardiac events during oncologic therapies are a major cause of morbidity and mortality [1-5]. Therapy-related and unrelated cardiac complications are increasing as patients’ survival improves. Myelodysplastic Syndrome (MDS) is a hematologic malignancy characterized by ineffective hematopoiesis that leads to peripheral-blood cytopenia, and patients with MDS progress to Acute Myeloid Leukemia (AML) [6,7]. Because MDS is a disease of the elderly, with the median onset occurring in the seventh decade of life [8], many comorbidities, including cardiac events, affect clinical outcomes [9]. In addition to chemotherapy for MDS, anemia [10] and infection [11] severely exacerbate Heart Failure (HF). Moreover, patients with MDS who have cardiovascular diseases, such as Congestive HF (CHF), have a significantly shorter survival than those with no cardiovascular conditions [11,12]. A report on causes of death of patients with MDS [13] revealed that HF accounts for 7.9% of all-cause death, and half of these deaths are disease unrelated.
Azacitidine, an inhibitor of DNA methyltransferase, exerts an antileukemic effect and is approved for treating MDS [14-18]. Currently, a single use of azacitidine has been shown to extend overall and leukemia-free survival compared with conventional cytotoxic therapies [19]. Several studies reported that azacitidine is well tolerated in elderly patients with MDS [8]. Common adverse effects of azacitidine are hematologic toxicity, gastrointestinal events, and general disorders such as malaise. Cardiovascular disease during azacitidine therapy is rarely reported in phase 1/2 multicenter studies of patients with MDS [20-22]. Moreover, only a few case reports of acute myocarditis induced by azacitidine and decitabine [23,24] have been reported. Conversely, a relatively high frequency of grade 3-4 cardiac events (8.4%-9.6%) is shown in multicenter studies for azacitidine-treated patients with AML [25,26].
For appropriate management during azacitidine therapy, analyzing the onset probability and risk factors of HF during azacitidine therapy is important. In this study, we investigated factors associated with HF onset and causes of death from a single institution’s experience. This is the first report of an HF survey during azacitidine therapy.
Material and Methods
Study design
We retrospectively analyzed 60 patients who were treated with azacitidine (75 mg/m2 per day for 7 days [54 patients] or 5 days [6 patients] every 28 days) from January 2009 to January 2017 in Chugoku Central Hospital. Evaluation criteria were (1) risk factors for HF onset during azacitidine therapy, (2) a scoring system to predict HF onset, (3) risk factors for death in the HF-onset group during azacitidine therapy. MDS and AML were diagnosed based on the 2008 World Health Organization classification. We used the international prognostic scoring system for patients with MDS. HF was diagnosed using Framingham Heart Study Criteria [27]. Death from HF was defined as death within 3 months after HF onset during azacitidine therapy. Red Blood Cell (RBC)-transfusion dependency was determined once every two weeks. Based on the results of univariate and multivariate analyses, we developed a scoring system to predict HF onset. We attributed 1 point for each risk factor that causes a significant difference in the two-year cumulative incidence of HF onset and divided the risk factors into three groups (low-, intermediate-, and high-risk groups) according to the total score. This study was approved by the institutional review board of Chugoku Central Hospital (No. 1807-06). The institutional review board approved this retrospective analysis in compliance with good clinical practice. Patients were offered the opportunity to opt out from this study by posting on the website and the poster on our hospital. This study was conducted in accordance with the Declaration of Helsinki.
Statistical analyses
We used Student’s t-test to compare continuous variables and Fisher’s exact test to compare categorical variables between the control and HF onset groups and the alive and dead subgroups in the HF onset group. Modified Odds Ratio (OR) was used partially. We used the Gray test for univariate analysis and fine-Gray test for multivariate analysis to confirm the cumulative incidence of the risk factors for HF onset. We used Kaplan-Meier survival curve and log-rank test for statistical comparison. Factors with p-values less than 0.05 were considered significant. All statistical analyses were performed using EZR statistical software (Saitama Medical Center, Jichi Medical University Saitama Medical Center, Saitama, Japan) [28].
Results
Description of patient characteristics
Patient characteristics at the start of treatment with azacitidine are shown in Table 1. Of the 60 patients, 15 (25%) developed HF. The mean age of the control group was 73.6 years and that of the HF onset group was 77.3 years (95% confidence interval [CI], -8.75-1.51; p=0.16). Fifty-three patients (88%) had MDS (40 vs. 13 patients; OR, 0.82; 95% CI, 0.12–9.53; p=1.00), and 7 patients (12%) had AML (5 vs. 2 patients; OR, 1.23; 95% CI, 0.10-8.67; p=1.00) in the control and HF onset groups. Significant differences in refractory cytopenia with multilineage dysplasia (OR, 6.55; 95% CI, 1.26-38.8; p=0.01) and refractory anemia with excess blasts-2 (OR, 0.07; 95% CI, 0.00-0.54; p‹0.01) were found between the control and HF onset groups. The number of azacitidine courses (6.96 vs. 7.40 courses; 95% CI, -4.46- 3.57; p=0.83) and total azacitidine cumulative dose (338 vs. 389*10 mg/m2; 95% CI, -237-136; p=0.59) were not different between the control and HF onset groups.
Mean
Control
HF onset
95% CIa
p value
Age at the start of azacitidine (mean, year)
73.6
77.3
-8.75
-
1.51
0.16
Total azacitidine (course)
6.96
7.4
-4.46
-
3.57
0.83
Total azacitidine cumulative dose (*10 mg/m2)
338
389
-237
-
136
0.59
Ferritin (ng/mL)
845
939
-492
-
304
0.64
BNP (pg/mL)
65.8
74.2
-56.1
-
39.3
0.73
EF (%)
70.6
68.6
-1.55
-
5.61
0.26
TRPG (mmHg)
24.7
19.2
-1.64
-
12.5
0.13
WT-1 mRNA (*103 copies/µgRNA)
13.3
40.1
-48.2
-
-5.5
0.01
*
Patient Number
Control, N=45 (%)
HF onset, N=15 (%)
Odds ratio
95% CIb
p value
Sex (male)
26
-58
12
(80)
1.67
0.34
-
11
0.73
Primary disease
MDS (WHO 2008 classification)
40
(89)
13
(87)
0.82
0.12
-
9.53
1.00
RCMD
4
(9)
6
(40)
6.55
1.26
-
38.8
0.01
*
RAEB-1
12
(27)
5
(33)
1.37
0.3
-
5.59
0.74
RAEB-2
23
(51)
1
(7)
0.07
0.00
-
0.54
<0.01
*
RCUD
1
(2)
1
(7)
3.07
0.04
-
252
0.44
AML
5
(11)
2
(13)
1.23
0.1
-
8.67
1.00
IPSS
Low
1
(2)
0
(0)
0.00
0.00
-
117
1.00
INT-1
11
(24)
3
(20)
0.78
0.12
-
3.68
1.00
INT-2
21
(47)
5
(33)
0.58
0.13
-
2.22
0.56
High
7
(16)
2
(13)
0.84
0.08
-
5.21
1.00
Past history
Heart disease
5
(11)
6
(40)
5.15
1.05
-
26.9
0.02
*
Lung disease
6
(13)
4
(27)
2.33
0.41
-
12.0
0.25
Renal failure
4
(9)
1
(7)
0.74
0.01
-
8.31
1.00
Heart+Lung disease
1
(2)
1
(7)
3.07
0.04
-
252
0.44
RBC-transfusion dependency
22
(49)
13
(87)
6.6
1.27
-
67.0
0.01
*
WT-1 mRNA (*103 copies/µgRNA) >50000
4
(9)
5
(33)
4.95
0.89
-
30.0
0.04
*
Results of upper column are continuous variables, and results of lower column are nominal variables of control (N=45) vs. HF onset (N=15) group. Laboratory data show the baseline at the start of first azacitidine therapy. RBC-transfusion dependency is determined at least once every two weeks during azacitidine therapy.
a: 95% CI of mean of differences from t-test, b: 95% CI of odds ratio from Fisher's test. *p <0.05.
BNP: Brain Natriuretic Peptide; EF: Ejection Fraction; TRPG: Transtricuspid Pressure Gradient; WT-1: Wilms' Tumor gene-1; MDS: Myelodysplastic Syndrome; WHO: World Health Organization; RCMD: Refractory Cytopenia with Multiline age Dysplasia; RAEB: Refractory Anemia with Excess Blasts; RCUD: Refractory Cytopenia with Unilineage Dysplasia; AML: Acute Myeloid Leukemia; IPSS: International Prognostic Scoring System; INT: Intermediate; RBC: Red Blood Cells
Table 1: Patient characteristics.
History of heart disease was significantly different between the control and HF onset groups (OR, 5.15; 95% CI, 1.05-26.9; p=0.02). No significant difference was found in baseline Brain Natriuretic Peptide (BNP) levels (65.8 vs. 74.2 pg/mL; 95% CI, -56.1-39.3; p=0.73) or Ejection Fraction (EF) ratio (70.6 vs. 68.6%; 95% CI, -1.55- 5.61; p=0.26). Lung disease (OR, 2.33; 95% CI, 0.41–12.0; p=0.25) and comorbidity of history of heart and lung disease (OR, 3.07; 95% CI, 0.04-252; p=0.44) had relatively high OR but showed no significant difference between the two groups. A significant difference was noted in RBC-transfusion dependency between the control and HF onset groups (OR, 6.60; 95% CI, 1.27-67.0; p=0.01). Baseline Wilms’ Tumor gene-1 (WT-1) mRNA (>50000 copies/μg RNA) was higher in the HF onset group (OR, 4.95; 95% CI, 0.89-30.0; p=0.04), and a significant difference was found in WT-1 mRNA value (13.3 vs. 40.1*103 copies/ μg RNA; 95% CI, -48.2 to -5.50; p=0.01).
Characteristics of patients who developed HF are shown in Table 2. Of the 15 patients, 9 patients recovered and 6 patients died from HF. The time to death from the start of azacitidine therapy was significantly shorter in the HF group than in the control group (median survival, 572 vs. 145 days; 95% CI, 457-794 vs. 39.0–390; p‹0.01) (Figure 3A). Infectious disease occurred in 11 patients (patients 1, 2, 4, 6, 7, and 10-15), and all patients who had an infection at HF onset had died (patients 10-15). Patients 1 and 12 had previous history of multi-drug combination chemotherapies before azacitidine therapy. Almost all patients (13/15) who had HF were RBC-transfusion dependent. Two patients (patients 2 and 3) who were not transfusion-dependent had Old Myocardial Infarction (OMI), CHF, and Chronic Kidney Disease (CKD) as comorbidities.
Patient
Age/Sex
Primary disease
IPSS risk group
WT-1 mRNA (copies/ µgRNA)
RBC-Transfusion dependency at the HF onset
Past history
Previous treatment
Azacitidine courses at the HF onset
Complication of HF
Outcome
1
78/female
MDS
RCMD
High
320
+
HT/Stroke/NTM
BHAC-DM
8
FN
Alive
2
72/male
MDS
RAEB-2
High
50
-
OMI
-
2
Sepsis
Alive
3
79/male
MDS
RCMD
Intermediate-1
1.4*103
-
CHF/CKD
-
5
-
Alive
4
79/male
MDS
RAEB-1
High
86*103
+
Stroke
-
2
FN
Alive
5
80/male
MDS
RAEB-1
Intermediate-2
2.8*103
+
Af
-
4
-
Alive
6
78/male
MDS
RCMD
Intermediate-2
480
+
Gastric cancer
-
6
FN
Alive
7
87/male
MDS
RAEB-1
High
85*103
+
-
CAG
2
FN
Alive
8
66/male
MDS
RCMD
Intermediate-2
50
+
COPD
-
1
-
Alive
9
93/female
MDS
RCMD
Intermediate-1
120*103
+
Gastric cancer
-
15
-
Alive
10
74/male
MDS
RAEB-1
Intermediate-2
50
+
IPF/AP/DM
-
9
Pneumonia
Death
11
80/male
MDS
RAEB-1
Intermediate-2
2.2*103
+
FL/HT
Rituximab, RB, CAG
4
FN
Death
12
67/male
AML
M0
NA
110*103
+
OMI/DM
CAG, SPAC, BHAC-DM, lenalidomide
2
FN
Death
13
77/female
MDS
RCUD
Intermediate-1
180*103
+
PE
CAG
3
FN
Death
14
72/male
MDS
RCMD
High
50
+
-
-
11
Pneumonia
Death
15
77/male
AML
M6
NA
740
+
DM/AAA
CAG
3
Pneumonia
Death
Primary disease, IPSS risk group, and WT-1 mRNA are at the first azacitidine therapy. Death from HF is defined as patients who died within 3 months after HF onset during azacitidine therapy.
IPSS: International Prognostic Scoring System; WT-1: Wilms’ Tumor Gene-1; RBC: Red Blood Cells; HF: Heart Failure; MDS: Myelodysplastic Syndromes; RCMD: Refractory Cytopenia with Multilineage Dysplasia; RAEB: Refractory Anemia with Excess Blasts; RCUD: Refractory Cytopenia with Unilineage Dysplasia; HT: Hyper Tension; NTM: Nontuberculous Mycobacteria; BHAC-DM: Behenoyl Cytosine Arabinoside, Daunorubicin; 6-Mercaptopurine; FN: Febrile Neutropenia; OMI: Old Myocardial Infarction; CHF: Chronic Heart Failure; CKD: Chronic Kidney Disease; Af: Atrial Fibrillation; CAG: Cytarabine; Aclarubicin; G-CSF; COPD: Chronic Obstructive Pulmonary Disease; IPF: Idiopathic Pulmonary Fibrosis; AP: Angina Pectoris; DM: Diabetes Mellitus; FL: Follicular Lymphoma; RB: Rituximab; Bendamustine; SPAC: Cytarabine Ocfosfate; PE: Pulmonary Embolism; AAA: Abdominal Aortic Aneurysm; NA: Not Applicable
Table 2: Patient characteristics of HF onset.
We experienced one case of azacitidine-induced pericarditis. In patient 8, HF developed on day 20 of the first azacitidine course. Echocardiography showed depressed EF and increased transtricuspid pressure gradient (the highest value was 68 mmHg), and this was suspected to be a complication of pulmonary hypertension. Computed tomography showed no signs of interstitial pneumonitis or acute lung injury, which were previously reported as rare adverse events of azacitidine [29,30]. Some pericardial effusion remained, but the amount of fluid did not allow for aspiration to be performed. The patient complained of continuous chest pain during the period of remaining pericardial effusion. Despite using diuretic agents, HF did not improve until about the 70th day after administering azacitidine. We suspected this HF was caused due to azacitidineinduced pericarditis. The manifestations, including cardiac and pleural effusion, gradually disappeared, but azacitidine was stopped due to the risk of HF recurrence.
History of heart disease, RBC-transfusion dependency, and high WT-1 mRNA titer can be risk factors for HF onset
Regarding the cumulative incidence of HF onset (Figure 1), past history of heart disease (cumulative incidence [%], 19.8 vs. 52.7; 95% CI, 9.51-32.7 vs. 14.2-81.1; p=0.01), RBC-transfusion dependency (cumulative incidence [%], 4.20 vs. 38.8; 95% CI, 9.53-32.7 vs. 14.2-81.1; p=0.02), and >50000 copies/μg RNA of WT-1 mRNA (cumulative incidence [%], 19.2 vs. 55.6; 95% CI, 9.23-31.8 vs. 16.2- 82.7; p=0.04) were significant risk factors at two years from the start of azacitidine therapy. The cumulative incidence results of other items measured at baseline are shown in Table 3. No significant difference was found regarding age, sex, total azacitidine course, history except for heart disease, and previous treatment. Overall survival of patients who underwent azacitidine therapy and with history of heart disease, RBC-transfusion dependency, and >50000 copies/μg RNA of WT-1 mRNA are shown in Figure 3 (B, C, D). No significant difference was noted in these risk factors, and we set death as a competing risk factor when we used the Gray test for assessing cumulative incidence of HF.
Cumulative incidence (%)
95% CIa
P value
Age
>70
30
16.4
-
44.8
<70
11.1
1.7
-
30.4
0.21
Sex
male
30.5
16.2
-
46.1
female
16.7
3.5
-
38.3
0.17
Total azacitidine (course)
>5
20
7.8
-
36.2
<5
32.5
14.5
-
52.1
0.43
Days of azacitidine administration
7 days
28.7
16.5
-
42.2
5 days
0.00
0.00
-
0.00
0.12
Past history
Lung disease-
24.8
12.8
-
38.3
Lung disease+
30
6.1
-
59.6
0.29
Heart+Lung disease-
24.8
13.9
-
37.4
Heart+Lung disease+
33.3
0.00
-
86.1
0.2
Renal failure-
27.8
16.0
-
40.9
Renal failure+
0.00
0.00
-
0.00
0.67
Malignant tumor-
25.3
12.8
-
39.9
Malignant tumor+
20.8
4.3
-
45.6
0.59
Hypertension-
21.5
9.8
-
36.2
Hypertension+
28.4
9.6
-
50.7
0.75
Collagen disease-
25.1
14.0
-
37.8
Collagen disease+
0.00
0.00
-
0.00
0.44
IPSS
Low
25.9
14.9
-
38.4
0.54
INT-1
14.3
2.0
-
37.9
0.49
INT-2
21.4
7.3
-
40.2
0.42
High
29.6
2.1
-
68.1
0.87
Previous treatment
26.4
10.4
-
45.6
0.43
Leukemia chemotherapy
33.3
13.1
-
55.3
0.22
Lymphoma chemotherapy
16.7
1.0
-
54.9
0.72
Other chemotherapy
20.0
0.00
-
62.1
0.77
BM cellularity (104/µl)
>5
21.9
9.4
-
37.8
<5
27.4
10.1
-
48.1
0.9
BM Blast (%)
>5
26.6
12.1
-
43.5
<5
24.4
9.3
-
43.2
0.76
PB Blast (%)
>5
20.0
2.6
-
49.2
<5
26.7
14.5
-
40.5
0.72
LDH (U/L)
>240
32.1
10.7
-
56.2
<240
22.9
11.0
-
37.4
0.66
eGFR (ml/min/body)
>50
26.3
14.8
-
39.4
<50
100
NA
-
NA
0.79
CRP (mg/dL)
>1
24.8
8.5
-
45.4
<1
26.0
12.4
-
41.9
0.78
Laboratory data show baseline at the start of azacitidine.
a: 95% CI of odds ratio from Fisher's test.
IPSS: International Prognostic Scoring System; INT: Intermediate; BM: Bone Marrow; PB: Peripheral Blood; LDH: Lactate Dehydrogenase; eGFR: estimate Glomerular Filtration Rate; CRP: C-Reactive Protein
Table 3: Univariate analysis of two-year cumulative incidence of HF onset from first azacitidine administration.
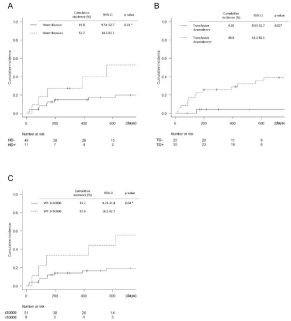
Figure 1: Cumulative incidence of HF onset.
In the multivariate analysis of cumulative incidence, history of heart disease (OR, 4.78; 95% CI, 1.51-15.2; p=0.01), RBC-transfusion dependency (OR, 5.21; 95% CI, 1.59-17.0; p=0.01), and >50000 copies/μg RNA of WT-1 mRNA (OR, 3.49; 95% CI, 1.15-10.5; p=0.03) had significantly higher OR (Table 4). Patients with MDS who already had cardiovascular disease, including CHF or chronic obstructive pulmonary disease, have significantly shorter survival than those with no comorbidities [11,12]; thus, lung disease and cardiac comorbidities of heart and lung diseases were also analyzed.
Odds ratio
95% CIa
P value
Heart disease
4.78
1.51
-
15.2
0.01
*
Lung disease
1.97
0.39
-
9.97
0.41
Heart+Lung disease
1.25
0.2
-
7.8
0.81
RBC-transfusion dependency
5.21
1.59
-
17
0.01
*
WT-1 >50000 (copy/µg RNA)
3.49
1.15
-
10.5
0.03
*
Risk
Criteria (points)
Cumulative incidence (%)
95% CIb
pvalue
Low
0.0
0.00
0.00
-
0.00
Intermediate
1.0
21.4
8.50
-
38.2
High
2.0 - 3.0
65.9
28.3
-
87.1
<0.01
*
Multivariate analysis is analyzed in fine Gray test. The risk groups of heart failure are characterized by summarizing the points of risk factors. The points of risk factors are defined as follows: heart disease, 1.0; RBC-transfusion dependency, 1.0; high WT-1 mRNA titer (>50000 copies/μg RNA), 1.0.
a: 95% CI of odds ratio from fine-gray test, b: 95% CI of cumulative incidence from gray test. *p<0.05.
RBC: Red Blood Cells; WT-1: Wilms' Tumor Gene-1.
Table 4: Multivariate analysis of risk factors and HF risk scoring system.
Scoring system for predicting HF during azacitidine therapy
Based on the results of univariate and multivariate analyses, we developed a scoring system to predict HF onset. Figure 2 shows the cumulative incidence of HF when patients were grouped using the risk scoring system. Two-year incidence of HF was significantly higher in patients with history of heart disease, RBC-transfusion dependency, and WT-1 mRNA >50000 copies/μg RNA. These three risk factors were assigned score points, and by combining the scores of the three risk factors, patients were stratified into three distinctive risk groups. The risk groups were as follows: low, 0.0; intermediate, 1.0; and high, 2.0 to 3.0. Figure 2 and Table 4 show the scoring system and cumulative incidence of HF in each group. These risk groups were found to have significantly different incidence of HF onset (cumulative incidence (%); 95% CI; low, 0.00%, 0.00-0.00; intermediate, 21.4%, 8.50-38.2; high, 65.9%, 28.3-87.1; p‹0.01). The use of the scoring system was helpful in separating risk groups to evaluate risk categories for predicting HF onset during azacitidine therapy.
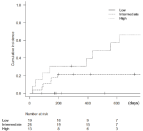
Figure 2: Cumulative incidence of HF-risk groups.
Infectious disease and previous heavy treatment can exacerbate HF and become lethal during azacitidine therapy.
Among 15 HF patients, 6 patients died within 3 months after HF onset. We then extracted the clinical characteristics that would become risk factors for exacerbating HF and death.
Univariate analysis of factors associated with death due to HF is shown in Table 5. There were significant differences in C-Reactive Protein (CRP) levels (5.30 vs. 14.6 mg/dL; 95% CI, -16.3 to -2.31; p=0.01) and infectious disease at HF onset (OR, 7.00; 95% CI, 2.65-18.4; p‹0.01) between the alive and dead subgroups. Previous chemotherapy, including anthracycline agents, was associated with a high odds ratio (OR, 6.00; 95% CI, 0.46–123; p=0.14), but was not a significant risk factor for death. Multiple prior regimens (>3) (OR, 10.6; 95% CI, 3.90–28.4; p‹0.01) were significantly associated with mortality.
Mean
Alive
Death
95% CIa
P value
Age at the start of azacitidine (mean, year)
79.1
74.5
-3.04
-
12.3
0.22
Total azacitidine cumulative dose (*10 mg/m2)
461
280
-297
-
659
0.43
Azacitidine Course at the HF onset
5.00
5.33
-5.05
-
4.39
0.88
Blood collection item at baseline
WBC (*103/µL)
2.55
2.51
-2.38
-
2.47
0.97
Hb (g/dL)
8.80
8.12
-0.86
-
2.22
0.36
Plt (*104/µL)
6.42
11.6
-15.6
-
5.35
0.31
Blood collection item at the HF onset
eGFR (ml/min/body)
78.6
60.7
-5.5
-
41.3
0.12
LDH (U/L)
197
614
-757
-
-168
0.02
*
CRP (mg/dL)
5.30
14.6
-16.3
-
-2.31
0.01
*
WT-1 mRNA (*103 copies/µgRNA)
7.45
34.9
-63.7
-
8.84
0.13
Patient Number
Alive, N=9 (%)
Death, N=6 (%)
95% CIb
p value
Sex (male)
7
(78)
5
(83)
1.4
0.06
-
101
1.00
Past history
Heart disease
3
(33)
3
(50)
1.91
0.15
-
26.1
0.62
Lung disease
3
(33)
1
(17)
0.42
0.01
-
7.47
0.6
Heart+Lung disease
1
(11)
1
(17)
1.55
0.02
-
141
1.00
Previous chemotherapy > 3 regimens
0
(0)
2
(33)
10.6
3.9
-
28.4
<0.01
*
Previous chemotherapy including Anthracycline
2
(22)
4
(67)
6.00
0.46
-
123
0.14
Infectious disease at the HF onset
6
(67)
6
(100)
7.00
2.65
-
18.4
<0.01
*
Exacerbation of blood collection item at the HF onset
WT-1 mRNA (*103 copies/µgRNA)
5
(56)
3
(50)
0.81
0.07
-
9.91
1.00
CRP (mg/dL)
7
(78)
6
(100)
4.33
1.62
-
11.6
<0.01
*
Results of upper column are continuous variables, and results of lower column are nominal variables of alive (N=9) vs. dead (N=6) group. Laboratory data show the baseline at the start of first azacitidine therapy and the result at the HF onset.
a: 95% CI of mean of differences from t-test, b: 95% CI of odds ratio from Fisher's test. *p <0.05.
HF: Heart Failure; WBC: White Blood Cell; Hb: Hemoglobin; Plt: Platelet; eGFR: estimate Glomerular Filtration Rate; LDH: Lactate Dehydrogenase CRP: C-Reactive Protein; WT-1: Wilms' Tumor gene-1
Table 5: Patient characteristics of HF onset group.
Discussion
We present a risk-scoring system to predict the development of HF during azacitidine therapy and risk factors exacerbating HF. This study is the first to analyze risk factors for HF during MDS therapy. A few studies have reported scoring systems that predict HF onset during chemotherapy, and each risk-scoring system could stratify patients by combinations of risk factors [31,32]. Such systems have not been reported in MDS management. Each factor was easily determined, and the subgroups were significantly related to the cumulative incidence of HF onset. The use of this risk-scoring system is potentially helpful in managing adverse cardiac events.
There has been an increasing interest in the importance of understanding cardio-oncology and cardiac toxicity during cancer therapy. Current oncologic agents and supportive therapies have improved the mortality rate of cancer patients. Therefore, therapyrelated cardiovascular complications, such as cardiomyopathy and CHF induced by radiation toxicity, cytotoxicity, and molecular target agents, have become one of the major problems associated with morbidity and mortality in surviving patients [1-5]. Azacitidine is a hypomethylating agent that can improve the overall survival of patients with MDS [21,22] and even patients with AML [33-35]. Investigation of cardiac events during azacitidine therapy is important for the management of patients with MDS or AML.
Common adverse events of azacitidine are hematologic toxicity, gastrointestinal events, and general disorders such as malaise [20-22]. MDS typically occurs in elderly people; thus, many comorbidities tend to occur during azacitidine therapy [8,9]. MDS patients who already had cardiovascular disease, including congestive HF or chronic obstructive pulmonary disease, had significantly shorter survival than patients without those conditions [11,12]. Patients with MDS or AML who were administered azacitidine in our hospital had relatively high HF onset (25%, 15/60) and mortality after onset (40%, 6/15) probability. Moreover, the time to death from the start of azacitidine therapy was significantly shorter in the HF group than in the control group (median survival, 572 vs. 145 days; 95% CI, 457- 794 vs. 39.0–390; p‹0.01) (Figure 3A). In our study, HF onset was frequently due to infections and developed comorbidities such as CHF or CKD (Table 2), but there was a characteristic case in which there were no other causes of HF except azacitidine (patient 8). Although the correlation between azacitidine and HF onset is unknown, some case series have reported azacytidine-induced myocarditis [23], interstitial pneumonitis [30,36-40], and acute lung injury [41]. Decitabine, which is also a hypomethylating agent, was reported to induce transient myocarditis [24]. Some studies have reported the development of noninfectious acute interstitial pneumonitis in association with azacitidine treatment in MDS patients [36-40]. In our study, there was a possibility that myocarditis occurred in patient 8 because he complained of chest pain and echocardiography showed pericardial effusion at the onset of HF, depressed EF, and increased transtricuspid pressure gradient, which were improved after stopping azacitidine therapy.
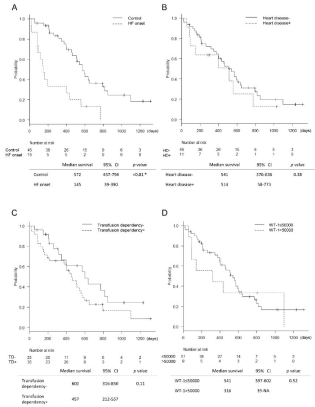
Figure 3: Overall survival from the first administration of azacitidine.
One of the reasons why HF as an adverse event of azacitidine has not been reported extensively in the past is thought to be that past prospective studies did not recruit patients with history of leukemia or uncontrolled CHF who did not meet the eligibility criteria [20- 22]. Our retrospective study included patients with leukemia (7/60 patients, 12%), patients who received leukemia chemotherapy (18/60, 30%), and patients with heart disease (11/60, 18%, Table 1). The adoption of azacitidine therapy has been expanded for more elderly people with cardiovascular comorbidities or leukemia. Overall survival was significantly shorter in the HF onset group than in the control group (Figure 3A). Therefore, analysis of HF onset probability and risk factors during azacitidine therapy is important.
The cardiac toxicity of azacitidine has not been observed in general, nor has it been well understood, but risk factors for HF during other chemotherapies have been reported in several previous studies. Anthracyclines, trastuzumab, and other biological and targeted agents are commonly reported to cause cardiac dysfunction, such as left ventricular dysfunction, myocardial ischemia, hypertension, and QTc prolongation [42]. Cumulative dose, history of radiation therapy, and multidrug combination therapy, such as combination with cyclophosphamide, were reported as risk factors for cardiac dysfunction [1]. In our study, the number of azacitidine courses and cumulative dose were not associated with HF onset (Table 1). Of the 60 patients, only three patients had history of prior irradiation but had no HF. None of the patients had received other chemotherapeutic agents such as cyclophosphamide during azacitidine therapy. If azacitidine itself is cardiotoxic, it is necessary to investigate other risk factors that cause cardiovascular events.
History of heart disease was a risk factor (OR, 4.78; 95% CI, 1.51-15.2; p=0.01) for HF onset in multivariate analysis. However, in univariate analysis, baseline BNP (65.8 vs. 74.2 pg/mL; 95% CI, -56.1-39.3; p=0.73) levels or EF ratio (70.6 vs. 68.6%; 95% CI, -1.55- 5.61; p=0.26) were not predictive of HF risk. These results suggest that even when HF was well controlled before azacitidine treatment, history of CHF could become a risk factor for HF onset following azacitidine therapy; this may be explained by the reduced cardiac reserve capacity shown by patients with a history of heart disease. Detailed assessments of cardiac biomarkers are necessary, but BNP or EF values were not a predicting factor for HF in our study. Further investigations of additional biomarkers, such as tumor necrosis factor-a, C-type natriuretic peptide, endothelin-1, cardiac troponin, and high-sensitivity C-reactive protein, are needed to evaluate cardiac metabolism and remodeling [43,44]. Using these items as an evaluation of cardiac reserve routinely could reduce the incidence of HF onset.
Multicenter studies of AML [25,26] showed 3-4 relatively highgrade events associated with the cardiac system (8.4%-9.6%). Our study demonstrated that high WT-1 mRNA titer was also a risk factor for HF (OR, 3.49; 95% CI, 1.15-10.5; p=0.03). The numeric value of 50000 copies/μg RNA was a high cutoff, indicating that a high tumor burden, such as in leukemia, could be a risk factor for HF. Previous reports showed that high levels of WT-1 mRNA are associated with worse outcomes in patients with AML [45,46] and MDS [47]. In our study, 5 patients in the HF onset group had a high tumor burden (WT- 1 mRNA higher than 50000 copies/μg RNA). These patients (patients 4, 7, 9, 12, and 13) developed infections, such as febrile neutropenia and pneumonia, before the onset of HF. With a high tumor burden and uncontrollable primary disease, they tended to acquire infections. Thus, they developed HF, although the mechanisms of HF onset with a high tumor burden remain unclear.
Our study revealed that RBC-transfusion dependency was an independent risk factor for HF onset in multivariate analysis (OR, 5.21; 95% CI, 1.59-17.0; p=0.01). Almost all patients (13/15, 93%) who had HF were RBC transfusion dependent. Two patients (patients 2 and 3) who were not transfusion-dependent had a history of OMI, CHF, and CKD. Patients with RBC transfusion-dependent MDS also have an increased risk of possible cardiac events due to both chronic anemia and iron overload [10,48], and the level of anemia and RBCtransfusion dependency are speculated to affect cardiac remodeling [49]. Although no significant difference was found between the control and HF onset groups in baseline serum ferritin value just before azacitidine therapy (845 vs. 939 ng/ml; 95% CI, -492–304; p=0.64, Table 1), a significant difference was noted between baseline and HF onset values (1051 vs. 1518 ng/ml; 95% CI, -754 to -179; p‹0.01) in 13 patients with RBC-transfusion dependency. These data suggest that RBC-transfusion dependency could induce iron overload. Thus, evaluation of serum ferritin value and iron chelation therapy is important to prevent cardiac dysfunction resulting from myocardial iron deposition, as reported in previous studies [50,51].
We analyzed the characteristics of the HF onset group and investigated the risk of death. Undergoing more than 3 previous courses of chemotherapy was a risk factor for death. Two patients (patient 11 and 12) who had more than 3 previous chemotherapy courses died of HF due to infectious disease caused by treatment for uncontrolled primary disease. In fact, infectious diseases were not only responsible for the development of HF but also exacerbated HF and resulted in death (OR, 7.00; 95% CI, 2.65-18.4; p‹0.01, Table 5). All patients in the dead subgroup had infectious disease before HF onset. Infectious disease and heavy treatment were confounding factors for death.
This study has some limitations. It was limited to a small group of patients in a single institution. Although it was impossible to perform statistical analysis for the factors correlated with death because of the small number of patients, these data can be referred to in managing patients who develop HF during azacytidine therapy. Further multicenter studies including a larger number of patients are needed.
Conclusion
Cardio-oncology is an important field in MDS management. To our knowledge, this study is the first to report an HF risk-scoring system during azacitidine therapy. The risk factors for HF were history of cardiac disease, RBC-transfusion dependency, and high WT-1 mRNA titer. Because HF onset is expected to become fatal and tends to shorten survival, appropriate management is needed.
References
- Han X, Zhou Y, Liu W. Precision cardio-oncology: understanding the cardiotoxicity of cancer therapy. npj Precision Oncology. 2017; 1.
- Sueta D, Hokimoto S, Utsunomiya D, Tabata N, Akasaka T, Sakamoto K, et al. New aspects of onco-cardiology. International journal of cardiology. 2016; 206: 68-70.
- Aleman BM, Moser EC, Nuver J, Suter TM, Maraldo MV, Specht L, et al. Cardiovascular disease after cancer therapy. EJC supplements: EJC: official journal of EORTC, European Organization for Research and Treatment of Cancer. 2014; 12: 18-28.
- Albini A, Pennesi G, Donatelli F, Cammarota R, De Flora S, Noonan DM. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardiooncological prevention. J Natl Cancer Inst. 2010; 102: 14-25.
- Abe J, Martin JF, Yeh ET. The Future of Onco-Cardiology: We Are Not Just “Side Effect Hunters”. Circulation research. 2016; 119: 896-899.
- Bejar R, Steensma DP. Recent developments in myelodysplastic syndromes. Blood. 2014; 124: 2793-2803.
- Garcia-Manero G. Myelodysplastic syndromes: 2015 Update on diagnosis, risk-stratification and management. Am J Hematol. 2015; 90: 831-841.
- Ritchie EK. Safety and efficacy of azacitidine in the treatment of elderly patients with myelodysplastic syndrome. Clin Interv Aging. 2012; 7: 165-173.
- Bammer C, Sperr WR, Kemmler G, Wimazal F, Nosslinger T, Schonmetzler A, et al. Clustering of comorbidities is related to age and sex and impacts clinical outcome in myelodysplastic syndromes. Journal of geriatric oncology. 2014; 5: 299-306.
- Oliva EN, Schey C, Hutchings AS. A review of anemia as a cardiovascular risk factor in patients with myelodysplastic syndromes. Am J Blood Res. 2011; 1: 160-166.
- Wang R, Gross CP, Halene S, Ma X. Comorbidities and survival in a large cohort of patients with newly diagnosed myelodysplastic syndromes. Leuk Res 2009; 33: 1594-1598.
- Naqvi K, Garcia-Manero G, Sardesai S, Oh J, Vigil CE, Pierce S, et al. Association of comorbidities with overall survival in myelodysplastic syndrome: development of a prognostic model. J Clin Oncol. 2011; 29: 2240- 2246.
- Nachtkamp K, Stark R, Strupp C, Kundgen A, Giagounidis A, Aul C, et al. Causes of death in 2877 patients with myelodysplastic syndromes. Ann Hematol. 2016; 95: 937-944.
- Silverman LR. Targeting hypomethylation of DNA to achieve cellular differentiation in Myelodysplastic Syndromes (MDS). The oncologist. 2001; 6: 8-14.
- Uchida T, Kinoshita T, Nagai H, Nakahara Y, Saito H, Hotta T, et al. Hypermethylation of the p15INK4B gene in myelodysplastic syndromes. Blood. 1997; 90: 1403-1409.
- Musto P, Maurillo L, Spagnoli A, Gozzini A, Rivellini F, Lunghi M, et al. Azacitidine for the treatment of lower risk myelodysplastic syndromes: a retrospective study of 74 patients enrolled in an Italian named patient program. Cancer. 2010; 116: 1485-1494.
- Khan C, Pathe N, Fazal S, Lister J, Rossetti JM. Azacitidine in the management of patients with myelodysplastic syndromes. Ther Adv Hematol. 2012; 3: 355-373.
- Kaminskas E, Farrell A, Abraham S, Baird A, Hsieh LS, Lee SL, et al. Approval summary: azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res. 2005; 11: 3604-3608.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009; 10: 223-232.
- Uchida T, Ogawa Y, Kobayashi Y, Ishikawa T, Ohashi H, Hata T, et al. Phase I and II study of azacitidine in Japanese patients with myelodysplastic syndromes. Cancer Sci. 2011; 102: 1680-1686.
- Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. Journal of clinical oncology: official Journal of the American Society of Clinical Oncology. 2006; 24: 3895-3903.
- Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002; 20: 2429-2440.
- Bibault JE, Cambier N, Lemahieu JM, Quesnel B, Auffret M, Rose C. Acute myocarditis induced by hypomethylating agents. J Clin Oncol. 2011; 29: e411-e412.
- De C, Phookan J, Parikh V, Nagrani T, Lakhani M, Forte F, et al. Decitabine induced transient cardiomyopathy: a case report. Clin Med Oncol. 2012; 6: 325-329.
- Pleyer L, Burgstaller S, Girschikofsky M, Linkesch W, Stauder R, Pfeilstocker M, et al. Azacitidine in 302 patients with WHO-defined acute myeloid leukemia: results from the Austrian Azacitidine Registry of the AGMT-Study Group. Ann Hematol. 2014; 93: 1825-1838.
- Pleyer L, Stauder R, Burgstaller S, Schreder M, Tinchon C, Pfeilstocker M, et al. Azacitidine in patients with WHO-defined AML - results of 155 patients from the Austrian Azacitidine Registry of the AGMT-Study Group. J Hematol Oncol. 2013; 6: 32.
- McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971; 285: 1441-1446.
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48: 452-458.
- Makita S, Munakata W, Watabe D, Maeshima AM, Taniguchi H, Toyoda K, et al. Azacitidine-induced acute lung injury in a patient with therapy-related myelodysplastic syndrome. J Int Med Res. 2017; 45: 886-893.
- Alnimer Y, Salah S, Abuqayas B, Alrabi K. Azacitidine-induced cryptogenic organizing pneumonia: a case report and review of the literature. Journal of medical case reports. 2016; 10: 15.
- Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014; 3: e000472.
- Chow EJ, Chen Y, Kremer LC, Breslow NE, Hudson MM, Armstrong GT, et al. Individual prediction of heart failure among childhood cancer survivors. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015; 33: 394-402.
- Tang L, Dolnik A, MacBeth KJ, Dombret H, Seymour JF, Minden MD, et al. Impact of Gene Mutations on Overall Survival in Older Patients with Acute Myeloid Leukemia (AML) Treated with Azacitidine (AZA) or Conventional Care Regimens (CCR). Blood. 2016; 128: 2859.
- Seymour JF, Dohner H, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. Azacitidine improves clinical outcomes in older patients with acute myeloid leukaemia with myelodysplasia-related changes compared with conventional care regimens. BMC cancer. 2017; 17: 852.
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Gattermann N, Germing U, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010; 28: 562-569.
- Sekhri A, Palaniswamy C, Kurmayagari K, Kalra A, Selvaraj DR. Interstitial lung disease associated with azacitidine use: a case report. Am J Ther. 2012; 19: e98-e100.
- Pillai AR, Sadik W, Jones PA, Thachil J. Interstitial pneumonitis-An important differential diagnosis for pulmonary sepsis in haematology patients. Leuk Res. 2012; 36: e39-e40.
- Adams CD, Szumita PM, Baroletti SA, Lilly CM. Azacitidine-induced interstitial and alveolar fibrosis in a patient with myelodysplastic syndrome. Pharmacotherapy. 2005; 25: 765-768.
- Hayashi M, Takayasu H, Tada M, Yamazaki Y, Tateno H, Tazawa S, et al. Azacitidine-induced pneumonitis in a patient with myelodysplastic syndrome: first case report in Japan. Internal medicine (Tokyo, Japan). 2012; 51: 2411- 2415.
- Hueser CN, Patel AJ. Azacitidine-associated hyperthermia and interstitial pneumonitis in a patient with myelodysplastic syndrome. Pharmacotherapy. 2007; 27: 1759-1762.
- Makita S, Munakata W, Watabe D, Maeshima AM, Taniguchi H, Toyoda K, et al. Azacitidine-induced acute lung injury in a patient with therapy-related myelodysplastic syndrome. J Int Med Res. 2017; 45: 886-893.
- Curigliano G, Cardinale D, Suter T, Plataniotis G, de Azambuja E, Sandri MT, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Am Oncl. 2012; 23: 155-166.
- Reis J. Reverse Cardiac Remodeling: A Marker of Better Prognosis in Heart. 2015; 104: 502-506.
- Regoli F, Regoli D, Moccetti T. Biological markers to predict cardiac resynchronization therapy effect. Circulation journal: official journal of the Japanese Circulation Society. 2014; 78: 2154-2156.
- Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, et al. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997; 90: 1217-1225.
- Cilloni D, Messa F, Arruga F, Defilippi I, Gottardi E, Fava M, et al. Early prediction of treatment outcome in acute myeloid leukemia by measurement of WT1 transcript levels in peripheral blood samples collected after chemotherapy. Haematologica. 2008; 93: 921-924.
- Jiang Y, Liu L, Wang J, Cao Z, Zhao Z. The Wilms’ tumor gene-1 is a prognostic factor in myelodysplastic syndrome: a meta analysis. Oncotarget. 2018; 9: 16205-16212.
- Malcovati L, Della Porta MG, Strupp C, Ambaglio I, Kuendgen A, Nachtkamp K, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classificationbased Prognostic Scoring System (WPSS). Haematologica. 2011; 96: 1433- 1440.
- Oliva EN, Dimitrov BD, Benedetto F, D’Angelo A, Nobile F. Hemoglobin level threshold for cardiac remodeling and quality of life in myelodysplastic syndrome. Leuk Res. 2005; 29: 1217-1219.
- Pinto V, Balocco M, Ambaglio I, Derchi G, Malcovati L, Forni GL. Iron overloadrelated heart failure in a patient with transfusion-dependent myelodysplastic syndrome reversed by intensive combined chelation therapy. Clinical case reports. 2015; 3: 952-954.
- Remacha AF, Arrizabalaga B, Villegas A, Duran MS, Hermosin L, de Paz R, et al. Evolution of iron overload in patients with low-risk myelodysplastic syndrome: iron chelation therapy and organ complications. Ann Hematol. 2015; 94: 779-787.