
Research Article
Ann Hematol Oncol. 2019; 6(5): 1249.
Efficacy of VRD (Bortezomib, Lenalidomide, and Dexamethasone) Consolidation Therapy and Maintenance Therapy with Immunomodulatory Agents (Thalidomide or Lenalidomide) after Autologous Peripheral Blood Stem Cell Transplantation in the Era of Bortezomib-Containing Induction Therapy: A Single Institution Experience
Tanimura A¹*, Hirai R¹, Nakamura M², Takeshita M¹, Togano T², Sekine R², Hagiwara S³ and Miwa A¹
¹Department of Hematology, Tokyo-Kita Medical Center, Tokyo, Japan
²Division of Hematology, National Center for Global Health and Medicine, Tokyo, Japan
³Division of Hematology, Tokyo Women’s Medical University, Tokyo, Japan
*Corresponding author: Akira Tanimura, Department of Hematology, Tokyo-Kita Medical Center. 4-17-56 Akabanedai, Kita-Ku, Tokyo, Japan
Received: March 18, 2019; Accepted: April 19, 2019;Published: April 26, 2019
Abstract
Autologous Stem Cell Transplantation (ASCT) for Newly-Diagnosed Multiple Myeloma (NDMM) has underwent recent improvements in combination with novel agents-containing induction and post-ASCT therapy. Since the approval of bortezomib for NDMM in Japan, we conducted the following regimen (BD arm) in transplant-eligible patients with NDMM: BD (bortezomib and dexamethasone) induction, ASCT, VRD consolidation, and maintenance therapy with Immunomodulatory Agents (IMIDs). The efficacy and safety of the BD arm were compared to those of patients treated with VAD (vincristine, doxorubicin, and dexamethasone) induction followed by ASCT (VAD arm) retrospectively. Thirty-three patients were treated with the BD arm, and 92 patients with the VAD arm. Thirty-one patients in the BD arm proceeded to ASCT. Thereafter, 23 and 17 patients received VRD consolidation and IMIDs maintenance therapy, respectively. The rates of complete response (CR)/=Very Good Partial Response (VGPR) after ASCT, consolidation, and maintenance therapy were 43%/61%, 76%/90% and 87%/93%, respectively. The response rates after ASCT did not differ between BD and VAD arms. The median progression-free survival (PFS) was 46.2 months (BD arm) and 30.6 months (VAD arm) (p=0.0106). The median Overall Survival (OS) was not-reached (BD arm) and 90.6 months (VAD arm) (p=0.0172).VRD consolidation and IMIDs maintenance therapies improved disease status after ASCT and prolonged PFS and OS.
Keywords: Multiple myeloma; Consolidation therapy; Maintenance therapy
Abbreviations
ASCT/SCT: Autologous Stem Cell Transplantation; NDMM: Newly-Diagnosed Multiple Myeloma; MM: Multiple Myeloma; BD: Bortezomib and Dexamethasone; VRD: Bortezomib; Lenalidomide; and Dexamethasone; IMIDs: Immunomodulatory Drugs; PFS: Progression-free Survival; OS: Overall Survival; VAD: Vincristine; Doxorubicin, and Dexamethasone; HD-CY: High Dose Cyclophosphamide; PR: Partial Response; VTD-PACE: Bortezomib; Thalidomide; Dexamethasone; Cis-Platin; Doxorubicin; Cyclophosphamide; and Etoposide; VGPR: Very Good Partial Response; CR: Complete Response; AE: Adverse Events; SPM: Second Primary Malignancy; MRD: Minimal Residual Disease; iFISH: Interphase Fluorescence in Situ Hybridization; DS: Durie-Salmon Stage; ISS: International Scoring System; ASCH/SCH: Autologous Stem Cell Harvesting; SD: Stable Disease, PD: Progressive Disease; NC: No Change; SCR: Stringent Complete Response
Introduction
Autologous Hematopoietic Stem Cell Transplantation (ASCT) prolongs the survival of patients with multiple myeloma (MM) who are up to 65 years of age, compared to conventional chemotherapy [1,2]. Because significant survival improvements can be afforded with new agents (proteasome inhibitors, immunomodulatory drugs, his tone deacetylase inhibitors and antibodies), up-front ASCT combined with new agent-containing induction therapies has become standard and the most important treatment option for patients with MM [3,4].
the introduction of new agents, VAD therapy (vincristine, doxorubicin, and dexamethasone) was the standard induction therapy for patients who were ASCT-eligible. Since the advent of bortezomib, bortezomi/dexamethasone-containing induction therapies became standard [3-9]. Because improvements in induction therapy response rate were projected to the prolong survival after ASCT, the effectiveness of 3-drug or 4-drug induction regimens containing bortezomib and IMIDs have been investigated.
Many studies of post-ASCT therapies (consolidation and/ or maintenance therapy) that contain new agents have been reported. Several reports indicate Progression-Free Survival (PFS) prolongation due to consolidation therapy [10-12]. However, only a few studies have shown overall survival benefits [13]. In maintenance therapy, there are concerns increased Second Primary Malignancy (SPM) frequencies after the long long-term use of Lenalidomide after high-dose and standard-dose melphalan [14-18]. With regard to the combination of agents with post-ASCT therapies, the patients who are suitable for therapy administration and the appropriate therapy durations await further determinations.
Our institute administered BD (bortezomib and dexamethasone) induction therapy, high-dose melphalan with stem cell support, VRD (bortezomib, lenalidomide and dexamethasone) consolidation therapy, and IMIDs maintenance therapy for the symptomatic and fit patients with MM patients who were under 66 years old. Here, we report the outcomes of 33 consecutive patients who received this regimen series and compare our results with those of a historical control study of VAD induction therapy followed by ASCTs from our institute.
Materials and Methods
Study design
This study was conducted at a single center, retrospectively. Response rates after BD induction therapy, stem cell harvesting by high dose cyclophosphamide + G-CSF, ASCT with high-dose melphalan, VRD consolidation therapy, and IMIDs maintenance therapy were accessed. The response rates, Progression-Free Survival (PFS) and Overall Survival (OS) of this study group (BD arm) were compared with those of a historical control group (VAD arm) who previously received VAD induction and ASCT in the same institute.
Patients
Patients were 65 years of age or younger, with newly diagnosed myeloma and without severe infections, uncontrolled diabetes, or severe organ dysfunctions. Patients were consecutively treated as candidates for ASCT in our hospital. From December 2009, these patients received BD induction therapy, high dose cyclophosphamidecontaining stem cell harvesting regimens (HD-CY + G-CSF), and high-dose melphalan supported with autologous hematopoietic stem cells, followed by VRD consolidation and IMIDs maintenance therapies. The historical control group was composed of patients, who had been treated in our hospital from January 2000 to November 2009 and received VAD therapy, HD-CY + G-CSF, or G-CSF alone harvesting regimens and ASCT, but did not receive planed post- ASCT therapy.
Treatments
BD arm: Four cycles of tri-weekly administration of bortezomib (1.3 mg/m2, subcutaneous injection on days 1, 4, 8, and 11) combined with oral dexamethasone (20mg, on days 1, 2, 4, 5, 8, 9, 11, and 12) were administered. The HD-CY containing regimen consisted of 2 doses of 2g/m2 cyclophosphamide, which were intravenously injected on 2 continuous days, followed by G-CSF. High dose melphalan (200mg/m2) was administered intravenously, divided into 2 days, and autologous stem cells were infused 2 or 3 days after the completion of melphalaninjection. As consolidation therapy, four cycles of triweek VRD administration (subcutaneously injected bortezomib (1.0mg/m2) on days 1, 4, 8, and 11, oral lenalidomide (15mg) days1- 14, and oral dexamethasone (20mg) days 1, 2, 4, 5, 8, 9, 11, and 12) were given. For IMIDs maintenance therapy, lenalidomide (5-10mg) or thalidomide (100mg), which were preferentially chosen by the attending physician, were given until Progressive Disease (PD) or unacceptable adverse events.
VAD arm (the historical control arm): Three or four cycles of 35- days of VAD, which consisted of continuous intravenous infusion of vincristine (0.4mg) days 1-4, doxorubicin (9mg/m2) days 1-4, and oral dexamethasone (40mg) days 1-4, 9-12, and 17-20) were administered. As a harvesting regimen, the HD-CY + G-CSF regimen was given from January 2000 to June 2009, and the G-CSF alone regimen was adapted from July 2009 to November 2009. ASCT was performed in the same way as described above for the BD arm. No planed posttransplant therapy was administered to the patients in the VAD arm.
Ethics
All patients from each arm provided written informed consent, which was performed according to the Helsinki Declaration principles.
Endpoints
The response rates after induction therapy, harvesting, and ASCT, and the survivals rates in the BD arm were compared with those of the VAD arm. The efficacy and toxicity of consolidation and maintenance therapies were evaluated in the BD arm.
Statistics
Differences between groups were evaluated using the Fisher’s exact test for categorical variables and the Mann-Whitney U-test for continuous variables. PFS was calculated as the time from treatment initiation to the first documentation of PD or death due to any cause. OS was calculated as the time from treatment initiation to death.PFS and OS were calculated using the Kaplan-Meier method. To evaluate the influence of the factors upon survival, the log-rank test was used.
All statistical analyses were performed with EZR (Saitama Medical Centre, Jichi Medical University), which is a graphical user interface of R (The R Foundation for statistical Computing, version 3.0.2) [19].
Results
Patient characteristics
Thirty-three and 92 patients in BD and VAD arms, respectively, were analyzed. There were no significant differences in patient backgrounds at the time of induction therapy initiation between both groups, except for the levels of hemoglobin and calcium, which were higher in the BD group than in the VAD group (Table 1).
VAD (92)
BD (33)
p value
Age (median, range)
58 (38-65)
56 (34-65)
0.343
Sex (Male/Female)
42/50
18 /15
0.421
IgG/A/D/E/BJP/other
58 /16 /4 /1 /12 /1a
22 /6 /1 /0 /3 /1b
0.783
ISS I/II/III (%)
33 (36) /36 (39) /23 (25)
10 (30) /14 (42) /9 (27)
0.872
DS stage III(%)
59 (64)
20 (61)
0.94
Albumin (g/dl, mean, SD)
3.6 (0.66)
3.5 (0.6)
0.622
Creatinine (mg/dl, median, range)
0.78 (0.42-7.02)
0.81 (0.55-5.01)
0.237
Calcium (mg/dl, median, range)
9.25 (7.2-16.3)
9.8 (8.5-12.3)
0.008
Hemoglobin (g/dl, mean, SD)
9.1 (2.1)
10.6 (1.7)
0.026
Normal karyotype /othersc
68/15
27/5
0.609
Observation (months, median, range)
78.8 (0.5-204.8)
44.3 (3.2-75.2)
<0.001
aIgG+IgA, bnon secretary, cany karyotype abnormalities without monosomy Y in male patients, Bd
bortezomib + dexamethasone therapy, DS Durie-Salmon, ISS International staging system, VAD
vincristine + doxorubicin + dexamethasone therapy
Table 1: Patient characteristics.
Clinical course of the BD arm
The median follow-up time was 44.3 months in the BD arm, and that of the VAD arm was 78.8 months (p < 0.001). Thirtyone patients of 33 patients in the BD arm proceeded to ASCT. Twenty-three patients received VRD consolidation therapy. Eight patients who did not receive VRD consolidation therapy had severe peripheral neuropathy, due to BD induction therapy, which resulted in cessation of further bortezomib treatment. These patients received lenalidomide/dexamthasone (RD) consolidation or proceeded to IMIDs maintenance therapy. Seventeen patients received IMIDs maintenance therapy (thalidomide 9, lenalidomide 8).
Nine patients (27%) had less than a partial response (PR) after induction therapy. Seven of whom received the VTD-PACE regimen (bortezomib, thalidomide, dexamethasone, cis-platin, doxorubicin, cyclophosphamide, and etoposide) as salvage therapy (20).Six of these seven patients achieved a PR or better, and successfully underwent stem cell harvesting by the VTD-PACE regimen, followed by G-CSF, just after VTD-PACE-related myelosuppression. One of these seven patients, from whom we failed to harvest sufficient stem cells by VTD-PACE, successfully underwent remobilization using plerixafor. Consequently, 31 patients successfully underwent stem cell harvesting and proceeded to ASCT (94%) (Figure 1).
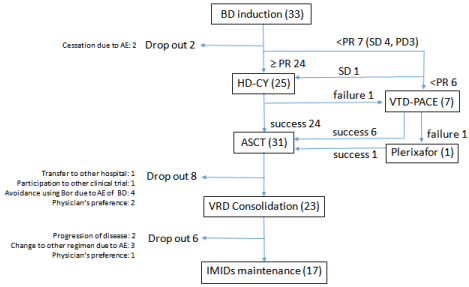
Figure 1: Clinical course of BD arm.
ASCT: Autologous Stem Cell Transplant; BD: Bortezomib + Dexamethasone
Therapy; HD-CY:High-Dose Cycophospamide; IMIDs: Immunomodulatory
Drugs; PD: Progressive Disease; PR: Partial Response; SD: Stable Disease;
VRD: bortezomib + lenalidomide + dexamethasone; VGPR: Very Good
Partial Response; VTD-PACE: bortezomib, thalidomide, dexamethasone,
cis-platin, doxorubicin, cyclophosphamide and etoposide.
Response rates
There were no statistically significant differences in the response rates after induction therapy, harvesting, and ASCT between the BD and VAD arms (Table 2). Among the 23 patients who had VRD consolidation therapy, 21 were evaluated, and their =VGPR and CR rates after consolidation therapy were 90% and 76%, respectively. Among the 17 patients who received maintenance therapy, the responses of were evaluated during maintenance, and the best =VGPR and CR rates were 93% and 87%, respectively. (Figure 2).
Response
VAD (92)
BD(33)
p-value
After Induction therapy
CR
7%
6%
>0.999
= VGPR
25%
18%
0.481
= PR
87%
73%
0.1
After ASCH
CR
10%
12%
0.743
= VGPR
28%
36%
0.387
PR
77%
88%
0.216
After ASCT
CR
33%
48%
0.141
= VGPR
47%
61%
0.224
= PR
80%
94%
0.096
BD bortezomib + dexamethasone therapy, CR complete response, DS Durie-Salmon, ISS International staging system, ASCH autologous stem cell harvesting, ASCT autologous stem cell transplant, PR partial response, VAD vincristine + doxorubicin + dexamethasone therapy, VGPR very good partial response.
Table 2: Comparison of response rates.
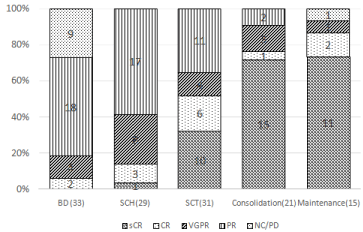
Figure 2: Response of BD arm.
BD: bortezomib + dexamethasone therapy; CR: Complete Response; NC: No
Change; PD: Progressive Disease; PR: Partial Response; SCH: Stem Cell
Harvesting; sCR: stringent CR; SCT: autologous stem cell transplant; VGPR:
Very Good Partial Response.
Survivals
The median PFS of the BD arm was significantly longer than that of the VAD arm (46.2 vs. 30.6 month, respectively, p=0.0106). In addition, the median OS of the BD arm was significantly longer than that of the VAD arm (not reached vs 90.6 months, respectively, p=0.0172) (Figure 3a and b). The median OS of patients with abnormal karyotypes was shorter than that of patients with normal karyotypes in the VAD arm; however, no significant differences between patients with abnormal or normal karyotypes were detected the in BD arm (Figure 4a and b).
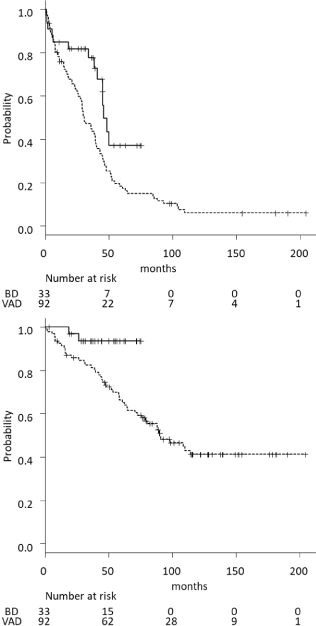
Figure 3: PFS and OS.
(a) Median PFS. BD arm vs. VAD arm: 46.2 months vs. 30.6months
(p=0.0106). (b) Median OS. BD arm vs. VAD arm: not reached vs. 90.6
months (p=0.0172). BD bortezomib + dexamethasone therapy, PFS
progression-free survival, OS overall survival, VAD vincristine + doxorubicin
+ dexamethasone therapy.
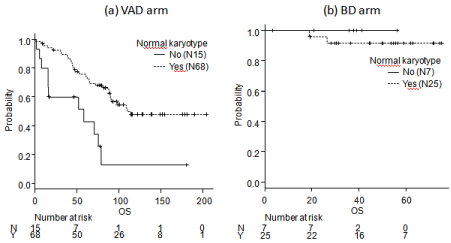
Figure 4: OS comparison of normal karyotype with other karyotypes in each arms.
(a) VAD arm, Yes vs. No: 114.7 months vs. 58.5 months, p=0.000622. (b) BD arm, Yes vs. No: Not reached vs. not reached, p=0.435. BD: bortezomib +
dexamethasone therapy; OS: Overall Survival; VAD: vincristine + doxorubicin + dexamethasone therapy.
Toxicity of consolidation and maintenance therapies
Seven of the 23 patients who received VRD consolidation therapy completed the four planned cycles of VRD without significant Adverse Events (AE). However, nine patients were forced to quit the
consolidation therapy due to transient AEs and resumed the therapy after AE improvement. Seven patients discontinued consolidation therapy due to severe AEs and proceeded to maintenance therapy without re-starting the consolidation therapy (2 thrombocytopenia, 1 pneumonia, 1 ileus, 1 peripheral neuropathy, 1 fatigue, and 1 leukocytopenia). Four patients required hospitalization due to severe AEs (2 pneumonia, 1 enterocolitis, and 1 ileus) (Table 3).
Adverse events
= G3 (%)
Leukocytopenia
2 (9)
Thrombocytopenia
3 (13)
Pneumonia
3 (13)
Upper respiratory infection
3 (13)
Enterocolitis
1 (4)
Ileus
1 (4)
Fatigue
1 (4)
Peripheral neuropathy
1 (1)
Table 3: Adverse events of VRD consolidation therapy (N23).
Seventeen patients received IMIDs maintenance therapies (9 thalidomide, 8 lenalidomide). The median maintenance therapy duration was 23 months (range: 5-58 months). Four patients discontinued maintenance therapy due to AEs (2 PN and 2 fatigue), and four patients discontinued due to disease progression. Nine patients were continuing maintenance therapy at the data cut-off time (2017/7/31) (Table 4).
Adverse events
Any grade (%)
= Grade 3 (%)
Peripheral neuropathy
4 (24)
1 (6)
Fatigue
2 (12)
1 (6)
Constipation
1 (6)
-
Upper respiratory infection
1 (6)
-
Skin rash
1 (6)
-
Thrombocytopenia
1 (6)
-
Table 4: Adverse events of IMIDs maintenance therapy (N17).
Salvage therapies of the VAD arm
In the VAD arm, 83 patients (90%) proceeded to ASCT and 60 patients received novel agents after ASCT (thalidomide 33, lenalidomide 20, bortezomib 26, and carfilzomib 1). Thalidomide was administered to 12 patients as maintenance therapy.
Primary second malignancy
The total observation time was 6718 months for the 92 patients in the VAD arm, and 1282 months for the 33 patients in the BD arm. Four second primary malignancies (2 breast cancers, 1acute myeloid leukemia, and 1 Hodgkin lymphoma) were observed in the VAD arm, and one incidence of prostate cancer in was observed in the BD arm. The incidence rates in the VAD and BD arms were 0.7145 (100/ person-year) and 0.8683 (100/person-year), respectively (Table 5).
Observational person-year
SPM
Incidence Rate (100/ person-year)
95% confidence interval
All
675
5
0.7407
0.3164-1.7341
VAD arm
560
4
0.7143
0.2777-1.8367
BD arm
115
1
0.8696
0.1534-4.9256
BD bortezomib + dexamethasone therapy, SPM second primary malignancy, VAD vincristine + doxorubicin + dexamethasone therapy
Table 5: .BD bortezomib+ dexamethasone therapy, SPM second primary malignancy, VAD vincristine + doxorubicin + dexamethasone therapy.
Discussion
For patients with NDMM, ASCT has verified superiority over conventional chemotherapy [1,2]. Novel agents have improved the survival of patients with refractory/relapsed MM and NDMM. Through combining novel agent-containing induction and posttransplant regimens, response and survival rates after ASCT have shown recent improvements [3-9,21.22]. Although bortezomib and dexamethasone have become the standardized agents for induction regimens, the most suitable regimen remains unknown.
Current post-transplantation therapies generally involve consolidation and maintenance therapies [12, 23]. Consolidation therapy is commonly used to improve responses after ASCT, over alimited period (6-12 months). Theoretically, maintenance therapies are designed to maintain responses after consolidation or ASCT regimens. In most reports, the agents that are used in maintenance therapies are orally administered, and are associated with minimal adverse events, since maintenance therapies typically last for at least 2-3 years, until PD.
Many consolidation regimens have been evaluated across multiple institutions: thalidomide and prednisolone [24], thalidomide + dexamethasone [25] (25), bortezomib alone [10,11], bortezomib + thalidomide + dexamethasone [25], lenalidomide + dexamethasone [14], bortezomib + lenalidomide + dexamethasone [3,26,27], and so on. Almost all of these studies reported that consolidation regimen efficacy deepens response rates after consolidation therapy. However, a small number of reports have directly compared of consolidation and no consolidation arms [10,11,24,27,28]. One study reported PFS and OS improvements by thalidomide consolidation + prednisolone (PSL) maintenance therapy, compared to PSL maintenance alone. In this study, most induction therapies consisted of conventional chemotherapies, with the exception of those for eight patients who received thalidomide [24]. Two studies compared bortezomib consolidation with no consolidation therapy [10,11], and in both studies, PFS improvements were observed in the consolidation arm, but no improvements in OS were identified. In both studies, no maintenance therapies were administered after consolidation. Only two studies directly compared consolidation + lenalidomidemaintenance with lenalidomide maintenance alone [27,28]. Found that two cycles of RVD consolidation improved PFS but not OS [27]. Reported that both four cycle-VRD and second ASCT consolidation therapies did not prolong PFS and OS [28]. A meta-analysis evaluated the six phase II and eight phase III studies, which compared consolidation + lenalidomide maintenance with lenalidomide maintenance alone. Consolidation therapies deepened response rates but did not improve PFS and OS [13].
With regard to maintenance therapy, many clinical studies have verified the efficacy and safety of thalidomide maintenance [29-34]. These studies reported that thalidomide maintenance improved PFS, mainly in patients who did not have poor prognostic cytogenetic abnormalities. However, only one study indicated the prolongation of OS [29]. Furthermore, one study reported that patients with myeloma and poor cytogenetic abnormalities did not benefit from thalidomide maintenance therapy [32].Two meta-analyses reported the PFS improvements by thalidomide maintenance therapy [35,36], one study reported the improvement of OS, while another study did not verify OSimprovement [35]. All studies that were analyzed in both meta-analyses did not include novel agent-containing induction therapies, and transplant-ineligible patients were included [36].
The efficacy of lenalidomide maintenance therapy has been analyzed in the post-ASCT setting, after novel agent-containing induction therapies [22,14,15,37]. All four studies that included novel-agent containing induction therapies and directly compared lenalidomide maintenance with no maintenance therapy reported that lenalidomide maintenance improves PFS, but only one study verified the prolongation of OS by lenalidomide maintenance therapy [15]. A meta-analysis of the above three studies identified both PFS and OS improvements by lenalidomide maintenance therapy [17].
Our institute initiated BD induction regimens in January 2010. The response rates after induction and ASCT showed no improvements, compared to those of patients in the VAD arm in our institute. In our institute, patients who did not obtain partial responses by BD induction regimens received VTD-PACE, as rescue and mobilizing regimens, and the majority of these patients successfully underwent stem cell harvesting and proceeded to ASCT. It is conceivable that VTD-PACE provides a useful rescue regimen for poor responders to induction therapy. Based on this result, BD induction might be insufficient, and 3 or 4-drug regimens that add one or two IMIDs and monoclonal antibodies to the BD backbone might be needed in the future.
At our institute, four cycles of VRD consolidation therapy are given after ASCT. The VRD consolidation therapy dosage is based on a phase 1 study that identified suitable VRD combination therapy dosages for patients with relapsed/refractory MM [38]. However, because we observed that four patients required hospitalized due to severe adverse events, four cycles of VRD consolidation therapy might be an overload for post-transplant patients. of the 21 evaluable patients, 19 (90%) and 16 (76%) reached =VGPR and CR after consolidation, with improved response rates of 65% (=VGPR) and 52% (CR) after ASCT.
Regarding to IMIDs maintenance therapy, attending physician s preferentially chose thalidomide or lenalidomide. The median maintenance therapy interval was 23 months (range: 5-58 months), and the best response rates during maintenance therapy were 93% (=VGPR) and 87% (CR). Four patients discontinued maintenance therapy due to AEs, four discontinued due to disease progression, and nine were continuing maintenance therapy at the data cut-off (2017/7/31). It is conceivable that the maintenance therapies of both immunomodulatory agents were effective and safe.
Although statistically significant response rate improvements immediately after ASCT were not observed in the BD arm, compared to the VAD arm, PFS and OS were longer in the BD arm than in the VAD arm. It is conceivable that response rate improvements after consolidation and maintenance therapies in the BD arm might induce longer survival periods.
The risk of increasing SPM after high-dose melphalan followed by long term lenalidomidemaintenance was reported by two phase III studies [14,15]. Another phase III study reported that lenalidomide maintenance did not increase the risk of SPM [22]. The increasing risk of SPM was verified by a meta-analysis that evaluated these three phase III studies [17]. The risk of death from MM significantly decreases after lenalidomide maintenance therapy, and the benefit obtained by lenalidomide maintenance surpassed the risk of death from SPM [17,39]. However, once a SPM occurs, it is devastating to the patient, and therefore physicians should carefully consider whether or not patient should receive lenalidomide maintenance therapy after ASCT or consolidation [39].
The advent of novel agents has improved CR rates and deepened responses. Highly sensitive methods for detecting Minimal Residual Disease (MRD), such as multi-color flow cytometry, ASO-RQ-PCR, and next generation sequencing are being introduced into clinical practice [40,41]. Through the application of these methods, patients with positive MRD who require post-ASCT therapies can avoid under treatment, and patients with negative MRD can avoid overtreatment.
ASCT combined with novel agent-including induction and posttransplant therapies has been verified to overcome the poor prognoses of high risk Cytogenetic Abnormalities (CA) that are detected by conventional karyotyping and I FISH. We did not analyze the impact of CA detected by I FISH, because the I FISH assay has not been tested for patients in the VAD arm, and positive results were detected in only a small number of the patients in the BD arm. The hyper diploid karyo type has been reported to indicate standard prognosis; however, in our analysis, the hyper diploid karyotype was associated with worse survival than the normal karyotype (data not shown). In the VAD arm, the OS of patients with abnormal karyotypes was shorter than that of those with normal karyotypes. In the BD arm, the OS of patients with abnormal karyotypes was not inferior to that of those with normal karyotypes. These results might indicate that our novel agent-containing ASCT program overcomes the poor risk of karyotype abnormalities. However, this result must be cautiously interpreted because, in this study population, the patient number was small, I FISH examination was not evaluated, and the observation period was short.
Conclusion
Novel agent-containing post-ASCT therapies have the potential to improve response rates and survival. However, the suitable regimens, appropriate durations, and suitable candidates for these therapies remain unknown. These variable need to be resolved by larger prospective trials that include proper risk stratifications and MRD assessments.
References
- Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Eng J Med. 1996; 335: 91-7.
- Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Eng J Med. 2003; 348: 1875-83.
- Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Eng J Med. 2017; 376: 1311-20.
- Rosinol L, Kumar S, Moreau P, Cavo M. Initial treatment of transplant-eligible patients in multiple myeloma. Expert Rev Hematol. 2014; 7: 43-53.
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol. 2010; 28: 4621-9.
- Rosinol L, Oriol A, Teruel AI, Hernandez D, Lopez-Jimenez J, de la Rubia J, et al. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012; 120: 1589-96.
- Sonneveld P, Schmidt-Wolf IG, van der Holt B, El Jarari L, Bertsch U, Salwender H, et al. Bortezomib induction and maintenance treatment in patients with newly diagnosed multiple myeloma: results of the randomized phase III HOVON-65/ GMMG-HD4 trial. J Clin Oncol. 2012; 30: 2946-55.
- Mai EK, Bertsch U, Durig J, Kunz C, Haenel M, Blau IW, et al. Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia. 2015; 29: 1721-9.
- Cavo M, Pantani L, Pezzi A, Petrucci MT, Patriarca F, Di Raimondo F, et al. Bortezomib-thalidomide-dexamethasone (VTD) is superior to bortezomib-cyclophosphamide-dexamethasone (VCD) as induction therapy prior to autologous stem cell transplantation in multiple myeloma. Leukemia. 2015; 29: 2429-31.
- Mellqvist UH, Gimsing P, Hjertner O, Lenhoff S, Laane E, Remes K, et al. Bortezomib consolidation after autologous stem cell transplantation in multiple myeloma: a Nordic Myeloma Study Group randomized phase 3 trial. Blood. 2013; 121: 4647-54.
- Christian Straka, Martin Vogel, Jürgen Müller, Martin Kropff, Bernd Metzner, Christian Langer, et al., editors. Results from two phase III studies of bortezomib (BTZ) consolidation observation (OBS) post-transplant in patients (pts) with Newly Diagnosed Multiple Myeloma (NDMM). J Clin Oncol. 2015; 33 (Suppl 15): 8511.
- Mohty M, Richardson PG, McCarthy PL, Attal M. Consolidation and maintenance therapy for multiple myeloma after autologous transplantation: where do we stand? Bone Marrow Transplant. 2015; 50: 1024-9.
- Al-Ani F, Louzada M. Post-transplant consolidation plus lenalidomide maintenance vs lenalidomide maintenance alone in multiple myeloma: A systematic review. Eur J Haematol. 2017; 99: 479-88.
- Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T, et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Eng J Med. 2012; 366: 1782-91.
- McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Eng J Med. 2012; 366: 1770-81.
- Jones JR, Cairns DA, Gregory WM, Collett C, Pawlyn C, Sigsworth R, et al. Second malignancies in the context of lenalidomide treatment: an analysis of 2732 myeloma patients enrolled to the Myeloma XI trial. Blood Cancer J. 2016; 6: e506.
- McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017; 35: 3279-89.
- Palumbo A, Bringhen S, Kumar SK, Lupparelli G, Usmani S, Waage A, et al. Second primary malignancies with lenalidomide therapy for newly diagnosed myeloma: a meta-analysis of individual patient data. Lancet Oncol. 2014; 15: 333-42.
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
- Barlogie B, Anaissie E, van Rhee F, Haessler J, Hollmig K, Pineda-Roman M, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007; 138: 176-85.
- Gay F, Oliva S, Petrucci MT, Conticello C, Catalano L, Corradini P, et al. Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015; 16: 1617-29.
- Palumbo A, Cavallo F, Gay F, Di Raimondo F, Ben Yehuda D, Petrucci MT, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Eng J Med. 2014; 371: 895-905.
- Joks M, Jurczyszyn A, Machaczka M, Skotnicki AB, Komarnicki M. The roles of consolidation and maintenance therapy with novel agents after autologous stem cell transplantation in patients with multiple myeloma. Eur J Haematol. 2015; 94: 109-14.
- Kalff A, Kennedy N, Smiley A, Prince HM, Roberts AW, Bradstock K, et al. Thalidomide and prednisolone versus prednisolone alone as consolidation therapy after autologous stem-cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the ALLG MM6 multicentre, open-label, randomised phase 3 study. Lancet Haematol. 2014; 1: e112-9.
- Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010; 376: 2075-85.
- Roussel M, Lauwers-Cances V, Robillard N, Hulin C, Leleu X, Benboubker L, et al. Front-line transplantation program with lenalidomide, bortezomib, and dexamethasone combination as induction and consolidation followed by lenalidomide maintenance in patients with multiple myeloma: a phase II study by the Intergroupe Francophone du Myelome. Journal of Clinical Oncology. 2014; 32: 2712-7.
- Pieter Sonneveld, Meral Beksac, Bronno van der Holt, Meletios A. Dimopoulos, Angelo Michele Carella, Heinz Ludwig, et al., editors. Consolidation Followed By Maintenance Therapy Versus Maintenance Alone in Newly Diagnosed, Transplant Eligible Patients with Multiple Myeloma (MM): A Randomized Phase 3 Study of the European Myeloma Network (EMN02/HO95 MM Trial). Blood. 2016; 128: 242.
- Edward Stadtmauer A, Marcelo Pasquini C, Beth Blackwell, Kristin Knust, Asad Bashey, Steven Devine M, et al. Comparison of Autologous Hematopoietic Cell Transplant (autoHCT), Bortezomib, Lenalidomide (Len) and Dexamethasone (RVD) Consolidation with Len Maintenance (ACM), Tandem Autohct with Len Maintenance (TAM) and Autohct with Len Maintenance (AM) for up-Front Treatment of Patients with Multiple Myeloma (MM): Primary Results from the Randomized Phase III Trial of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0702 - StaMINA Trial). Blood. 2016; 128: LBA-1 [abstract].
- Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006; 108: 3289-94.
- Barlogie B, Pineda-Roman M, van Rhee F, Haessler J, Anaissie E, Hollmig K, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008; 112: 3115-21.
- Lokhorst HM, van der Holt B, Zweegman S, Vellenga E, Croockewit S, van Oers MH, et al. A randomized phase 3 study on the effect of thalidomide combined with adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010; 115: 1113-20.
- Morgan GJ, Davies FE, Gregory WM, Bell SE, Szubert AJ, Cook G, et al. Long-term follow-up of MRC Myeloma IX trial: Survival outcomes with bisphosphonate and thalidomide treatment. Clin Cancer Res. 2013; 19: 6030-8.
- Stewart AK, Trudel S, Bahlis NJ, White D, Sabry W, Belch A, et al. A randomized phase 3 trial of thalidomide and prednisone as maintenance therapy after ASCT in patients with MM with a quality-of-life assessment: the National Cancer Institute of Canada Clinicals Trials Group Myeloma 10 Trial. Blood. 2013; 121: 1517-23.
- Maiolino A, Hungria VT, Garnica M, Oliveira-Duarte G, Oliveira LC, Mercante DR, et al. Thalidomide plus dexamethasone as a maintenance therapy after autologous hematopoietic stem cell transplantation improves progression-free survival in multiple myeloma. Am J Hematol. 2012; 87: 948-52.
- Morgan GJ, Gregory WM, Davies FE, Bell SE, Szubert AJ, Brown JM, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012; 119: 7-15.
- Kagoya Y, Nannya Y, Kurokawa M. Thalidomide maintenance therapy for patients with multiple myeloma: meta-analysis. Leuk Res. 2012; 36: 1016-21.
- Graham Jackson H, Faith Davies E, Charlotte Pawlyn, David Cairns A, Alina Striha, Corinne Collett, et al. Lenalidomide Is a Highly Effective Maintenance Therapy in Myeloma Patients of All Ages; Results of the Phase III Myeloma XI Blood. 2016; 128: 1143.
- Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL, et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol. 2009; 27: 5713-9.
- Musto P, Anderson KC, Attal M, Richardson PG, Badros A, Hou J, et al. Second primary malignancies in multiple myeloma: an overview and IMWG consensus. Ann Oncol. 2017; 28: 228-45.
- Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016; 17: e328-46.
- Sherrod AM, Hari P, Mosse CA, Walker RC, Cornell RF. Minimal residual disease testing after stem cell transplantation for multiple myeloma. Bone Marrow Transplant. 2016; 51: 2-12.
Citation: Tanimura A, Hirai R, Nakamura M, Takeshita M, Togano T, Sekine R, et al. Efficacy of VRD (Bortezomib, Lenalidomide, and Dexamethasone) Consolidation Therapy and Maintenance Therapy with Immunomodulatory Agents (Thalidomide or Lenalidomide) after Autologous Peripheral Blood Stem Cell Transplantation in the Era of Bortezomib-Containing Induction Therapy: A Single Institution Experience. Ann Hematol Oncol. 2019; 6(5): 1249.