
Research Article
Ann Hematol Oncol. 2019; 6(7): 1256.
Newly Diagnosed Patients with Polycythemia Vera and Essential Thrombocythemia Have an Impaired Quality of Life Compared to Age-Matched Control Individuals
Pettersson H¹, Anderson L², Abelsson J¹, Samuelsson J³, Johansson P¹, Duncombe A4, Mesa R5, McMullin MF6 and Andréasson B¹*
¹Hematology Section, Specialist Medicine, NU Hospital Group, UK
²Centre for Public Health, Queen’s University Belfast, UK
³Department of Internal Medicine, Stockholm South Hospital, UK
4Department of Hematology, University Hospitals Southampton NHS Foundation Trust, UK
5UT Health San Antonio Cancer Center, San Antonio, USA
6Centre for Cancer Research and Cell Biology, Queen’s University Belfast, UK
*Corresponding author: Björn Andreasson, Specialist Medicine, NU Hospital Group, Uddevalla sjukhus, Fjällvägen 9, 45180 Uddevalla, UK
Received: April 08, 2019; Accepted: July 02, 2019;Published: July 09, 2019
Abstract
Purpose: The Myeloproliferative Neoplasms (MPN) are rare disorders with a prevalent and often severe symptom burden. The MPN- Symptom Assessment Form (SAF) is a well-documented and validated tool for measuring symptom burden in these patients. In this study we compared , symptom burden and overall Quality of Life (QoL) measured by the MPN-SAF, in 164 patients with newly diagnosed Polycythemia Vera (PV) or Essential Thrombocythemia (ET), 119 patients with elevated hematocrit and/or platelet count (non-PV/ET) without evidence of MPN and 495 healthy, age-matched control subjects.
Methods: The MPN-SAF is a 27-item questionnaire specifically tailored to MPN patients was sent to the patients and healthy control subjects, and returned by mail. MPN-SAF Total Symptom Score (TSS) and Brief Fatigue Inventory (BFI) were calculated from the MPN-SAF questionnaire. The scores of the three groups were compared to each other using the Mann-Whitney U Test.
Results: The control group had significantly lower means for all 27 items, measured by MPN-SAF, including TSS compared to the newly diagnosed PV/ ET patients, as well as compared to the non-PV/ET group. Surprisingly, the non- PV/ET group had a more severe symptom burden than the newly diagnosed PV and ET patients with higher scores for all items except weight loss.
Conclusions: This is to date the largest set of data on how age-matched healthy subjects score in MPN-SAF, for possible reference in future studies. Newly diagnosed PV and ET patients have significantly impaired QoL compared to the general population. Also, MPN-SAF does not distinguish MPN patients from patients with elevated blood parameters not due to MPN, but these patients should be further reviewed due to their substantial symptom burden.
Keywords: Polycythemia vera; Thrombocythemia; Quality of life
Abbreviations
MPNs: Myeloproliferative Neoplasms; PV: Polycythemia Vera; ET: Essential Thrombocythemia; MF: Myelofibrosis; QoL: Quality of Life; SAF: Symptom Assessment Form; Hct= Hematocrit; TSS: Total Symptom Score; BFI: Brief Fatigue Inventory
Introduction
The Philadelphia chromosome negative Myeloproliferative Neoplasms (MPNs) are rare clonal hematologic malignancies including Polycythemia Vera (PV), Essential Thrombocythemia (ET) and Myelofibrosis (MF) [1]. These entities are heterogeneous and have differences as to morbidity and prognosis, but also share features such as risk for vascular complications and transformation into acute leukemia [2-6]. The MPNs also share a prevalent and often severe symptom burden [7-9].
In recent years, several studies with focus on symptom burden and the Quality of Life (QoL) of MPN patients have been published. It is evident that MPN patients have impaired QoL and several instruments have been used to capture this [8,10-12]. The widely used instruments in cancer, such as the European Organization of Research and Treatment of Cancer Quality of Life Questionnaire Core model (EORTC QLQ-C30) and Functional Assessment of Cancer Therapy-Anemia (FACT-AN), do not fully reflect the specific symptoms which affect QoL in MPN patients [13,14]. To adequately profile the symptoms of MPN, the MPN-Symptom Assessment Form (SAF) was developed, including 27 items. This tool has been clinically validated and has been translated to several languages, to be used in international studies. International studies using MPN-SAF have illustrated an impaired QoL and substantial symptom burden in MPN patients both at diagnosis and during the course of the disease [14]. The symptom burden seems to be more severe in patients with MF over time [14], whereas PV patients were found to worst impairment of QoL at the time of MPN diagnosis [12].
Very few comparisons between QoL in patients with MPN and the general population have been done. To our knowledge, the only study presenting data including a control group is the one by Anderson et al, [11]. Herein, the MPN-SAF scores from 106 MPN patients from the UK and 1446 from the US were compared to the scores from 124 healthy control subjects. In all 27 MPN-SAF items the MPN patients scored significantly higher compared to the controls, i.e. had an increased symptom burden.
The aim of this study was to compare QoL, using MPN-SAF, in patients with newly diagnosed PV or ET with patients referred to a hematologic clinic due to elevated Hematocrit (Hct) and/or platelet counts who had no evidence of MPN and an age adjusted group of healthy individuals.
Methods
Patients and control subjects
The control individuals were recruited among relatives of patients and hospital staff. The only inclusion criteria were age =55 years and that they considered themselves healthy. The control group comprised 401 subjects from Sweden and 94 subjects from Great Britain, 291 were female and 204 were male. The British group of healthy individuals is a subgroup of a cohort earlier described by Anderson et al, [11], i.e. those with age =55 years.
The 119 patients with elevated Hct and/or elevated platelet counts, referred to as non-PV/ET, were recruited from three Swedish centers; Stockholm South Hospital, Uddevalla Hospital and Sahlgrenska University Hospital. At the time of referral the patient had an Hct over 48% in women and over 50% in men and/or a platelet count exceeding 450 x109/L. To be included in the study the patients with elevated Hct had to have a normal serum erythropoietin and absence of JAK2 V617F mutation. The patients with thrombocytosis had to have absence of JAK2 V617F mutation and a bone marrow biopsy without MPN features when no evident cause of platelet elevation found.
The control groups and the patients with high blood values were contacted per written letter with study information, consent form and MPN-SAF questionnaires. Age and gender were recorded.
The majority of patients with newly diagnosed ET (n=80) and PV (n=73) from Sweden were recruited from six hematology clinics, and are described in detail in a previously published study of Abelsson et al, [12]. In addition, eleven ET or PV patients were included from Uddevalla Hospital. All included patients had their diagnosis established according to the 2008 World Health Organization (WHO) Guidelines [15] and completed the survey as soon as the diagnosis was established.
Questionnaire
The MPN-SAF is a 27-item questionnaire specifically tailored to MPN patients. This instrument has been validated in 2012 and been translated to many languages. Each item was answered with a linear analog scale of 0-10, where 0 corresponded to absence of symptom and 10 to the worst possible. A high score in MPN-SAF would reflect a poor quality of life [13,14].
MPN-SAF Total Symptom Score (TSS) is a shorter form of the questionnaire which includes 10 items: fatigue, concentration problems, early satiety, inactivity, night sweats, itching, bone pain, abdominal discomfort, weight loss and fever [14].
Brief Fatigue Inventory (BFI) is a 9-item score included in MPNSAF [16]. BFI is an average score of 9 items reflecting fatigue and how it affects related activity [8].
Ethical consideration
The study was approved by the Office for Research Ethics Committee, Northern Ireland and by the Regional Ethical Board in Gothenburg, Sweden. All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients and control subject gave their informed consent.
Statistics
The mean scores and the standard deviation were calculated with standard statistical methods. The scores of the three groups were compared to each other using the Mann-Whitney U Test. A 95% confidence interval was chosen.
Results
The group of healthy controls included 401 Swedish and 94 British individuals with a mean age of 66.6 years. The group of 119 patients with non-PV/ET had a mean age of 61 years, which did not differ significantly when compared to the controls (p=0.158). On the other hand, the group of 164 newly diagnosed PV and ET patients was significantly older than both controls and the non-PV/ET patient group, mean age of 70.1 years, (p <0.001, for both).
The score for every MPN-SAF item were similar and the means did not differ significantly in the healthy controls from Sweden compared to the British, except the item addressing sexual problems where the Swedish controls had a mean score of 2.0 compared to 1.1 for the British subjects (p<0.001).
The group of 164 newly diagnosed PV and ET patients had significantly higher means in all MPN-SAF items, p =0.005, compared to the healthy controls, including BFI and TSS. Thus, the mean score was at least double in the PV/ET group in all MPN-SAF items except BFI, insomnia, sexual problems, cough, night sweats, bone pain and overall QoL. Detailed data are shown in (Table I) and illustrated in (Figure 1).
Because of the significant difference in mean age between the healthy controls and the PV/ET patients we also compared the MPNSAF scores for the control subjects with age >62 years. This group comprised 332 subjects and had a mean age comparable to the patients, 70.3±6.0 years. When this age-matched group was compared to the patients with newly diagnosed MPN all the highly significant differences (p =0.005) remained, except for the items addressing early satiety and sexual problems (p =0.025 and 0.053, respectively).
The MPN-SAF scores from the 119 non-PV/ET patients were compared with the scores from the healthy control group. In all items the control group had mean scores that were highly significantly lower (p<0.001, in all items). In all items in the questionnaire the non-PV/ET patients had at least a double mean value compared to the means of the controls, except for the questions about insomnia and sexual problems.
In spite of the fact that the group of patients with newly diagnosed PV/ET had a significantly higher mean age their mean MPN-SAF scores were lower for all items, except weight loss, when compared to the means for the non-PV/ET patients. Statistical significance was reached for BFI, early satiety, abdominal pain and discomfort, inactivity, numbness, night sweats, overall QoL and TSS. All mean scores and significance levels are shown in Table 1 and illustrated in (Figure 2).
A) Controls n=495
B) non PV/ET n=119
C) ET/PV n=164
Mean
SD
p
AvsC
Mean
SD
p
AvsB
Mean
SD
p
BvsC
Age
66.6
7.3
<0.001
61.0
15.5
0.158
70.5
12.8
<0.001
BFI
1.7
1.5
<0.001
3.7
2.6
<0.001
2.7
2.3
0.005
Early satiety
1.1
1.8
0.004
2.5
2.6
<0.001
1.8
2.5
0.019
Abdominal pain
0.4
1.1
<0.001
1.8
2.6
<0.001
1.1
2.0
0.036
Abdominal discomfort
0.6
1.3
<0.001
2.2
2.7
<0.001
1.3
2.0
0.009
Inactivity
0.9
1.6
<0.001
2.9
2.7
<0.001
2.0
2.4
0.011
Headache
0.6
1.3
<0.001
2.2
2.8
<0.001
1.8
2.5
0.406
Concentration problem
0.6
1.5
<0.001
2.3
2.9
<0.001
2.2
2.6
0.922
Dizziness
0.5
1.3
<0.001
2.3
2.8
<0.001
1.8
2.2
0.397
Numbness
0.8
1.6
<0.001
2.7
3.0
<0.001
1.9
2.5
0.031
Insomnia
1.7
2.2
0.005
3.2
3.3
<0.001
2.6
3.0
0.106
Sad mood
0.8
1.5
<0.001
2.6
2.9
<0.001
2.1
2.5
0.142
Sexual problem
1.8
2.7
0.001
3.4
3.7
<0.001
2.9
3.4
0.250
Cough
1.0
1.8
0.004
1.9
2.6
<0.001
1.3
1.9
0.072
Night sweats
0.9
1.7
<0.001
2.9
3.1
<0.001
1.7
2.4
0.002
Pruritus
0.6
1.4
<0.001
1.8
2.4
<0.001
1.7
2.5
0.769
Bone pain
0.7
1.6
<0.001
2.0
2.6
<0.001
1.3
2.1
0.058
Fever
0.1
0.4
<0.001
0.5
1.4
<0.001
0.3
1.0
0.558
Weight loss
0.2
0.9
<0.001
0.7
1.7
<0.001
0.9
1.9
0.399
Overall QoL
1.4
1.8
<0.001
3.6
2.8
<0.001
2.6
2.3
0.005
TSS
4.3
5.8
<0.001
13.0
11.1
<0.001
8.9
9.7
<0.001
Table 1: Symptom burden and Quality of Life measured with MPN-SAF in patients with newly diagnosed PV or ET, patients with high hemoglobin and/or platelets without MPN and a group of healthy individuals.
Discussion
The MPN-SAF is a well-documented and validated tool for measuring symptom burden and QoL in this group of patients. Several studies have highlighted the fact that patients with MPN have impaired QoL, both at the time of diagnosis as well as during the course of the disease [7-10,12-14]. One major limitation of these studies is the lack of a control group of age matched healthy individuals; only one study has presented a comparison between MPN patients and healthy individuals. Anderson et al, [11] reported a significantly impaired QoL for 106 MPN patients compared to 126 healthy control subjects from UK. In our study we recruited 401 healthy individuals from Sweden with age =55 years and pooled them with the 94 controls from the UK study in this age group. The symptom burden and QoL, measured by MPN-SAF, was similar in both control groups despite differing recruitment strategies and residency.
We found that the MPN patients scored significantly higher in every MPN-SAF item, including TSS, when compared to the healthy subjects (Figure 1). In fact, several items had at least double the mean score for the MPN patient group compared to controls; abdominal pain and discomfort, inactivity, headache, concentration, dizziness, numbness, sad mood, night sweat, pruritus, weight loss and TSS. Also, when a subset of the cohort of healthy individuals, with an age comparable to the MPN patients, was analyzed the significant differences between mean MPN-SAF scores remained, except for the item addressing sexual problems which might reflect that this is an increasing problem with age also in the general population. Thus, there is a significant correlation between increasing age and higher score for the question about sexual problems in control group (p<0.001).
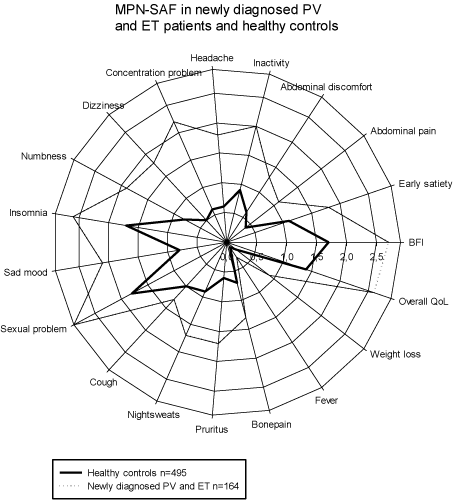
Figure 1: MPN-SAF in 164 newly diagnosed PV and ET patients and 495 healthy controls.
Surprisingly, in our study the patients a high Hct and/or platelet count without evidence of MPN had the most impaired QoL of the three investigated groups. These patients were not followed or further investigated at our hematological units, so the causes of blood count elevations are not completely known. We could speculate that several of the patients with elevated Hct have chronic pulmonary problems, which is well known to negatively influence QoL [17]. Many of the patients with thrombocytosis are likely to have chronic inflammatory disease and some may have other cancers, factors that give an impairment of QoL. Still, we did not expect these patients to report higher mean score for almost all MPN-SAF items (Figure 2).
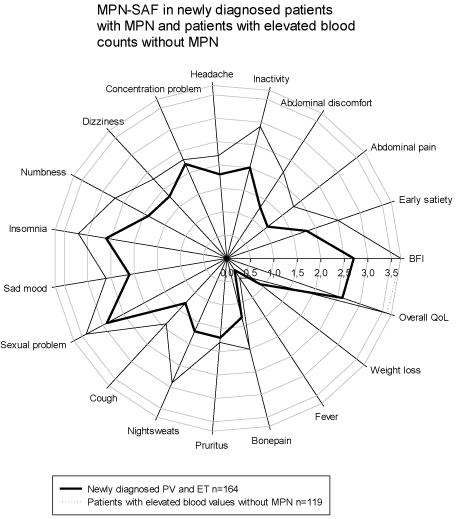
Figure 2: MPN-SAF in 164 newly diagnosed PV and ET patients and 119 patients with elevated blood values without MPN.
Conclusion
We conclude that patients with newly diagnosed PV or ET have significantly impaired QoL, measured with MPN-SAF, compared to healthy age-matched subjects. However, these QoL findings cannot discriminate patients with MPN from patients with secondary erythrocytosis and/or reactive thrombocytosis who tend to have worse symptom scores.
Acknowledgements
The authors thank the patients and controls who participated in this study. Also, thanks to the MOSAICC study team including Dr Glen James, Dr Frank de Vocht, Prof Lin Fritschi, Prof Mike Clarke.
Funding
This study was supported by grants from FOU, NU hospital group and funding from MPNVoice.
Availability of Data
Aggregated data are shown in (Table 1) and figures. Please contact authors for data requests.
Authors Contributions
HP participated in design of the study, statistical analysis and manuscript writing. LA participated in data collection and manuscript writing. JA participated in data collection. JS participated in data collection and manuscript writing. PJ participated in data collection. AD participated in data collection. RM participated in manuscript writing. MFM participated in data collection and manuscript writing. BA participated in design of the study, statistical analysis and manuscript writing. All authors read and approved the final manuscript.
Ethic’s Approval
The study was approved by the Office for Research Ethics Committee, Northern Ireland and by the Regional Ethical Board in Gothenburg, Sweden (Dnr 923-11). All participating patients and control subjects gave their informed consent to participate.
References
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117: 5019-5032.
- Abdulkarim K, Ridell B, Johansson P, Kutti J, Safai-Kutti S, Andreasson B. The impact of peripheral blood values and bone marrow findings on prognosis for patients with essential thrombocythemia and polycythemia vera. European journal of haematology. 2011; 86: 148-155.
- Tefferi A, Rumi E, Finazzi G, Gisslinger H, Vannucchi AM, Rodeghiero F, et al. Survival and prognosis among 1545 patients with contemporary polycythemia vera: an international study. Leukemia. 2013; 27: 1874-1881.
- Reikvam H, Tiu RV. Venous thromboembolism in patients with essential thrombocythemia and polycythemia vera. Leukemia. 2012; 26: 563-571.
- Kundranda MN, Tibes R, Mesa RA. Transformation of a chronic myeloproliferative neoplasm to acute myelogenous leukemia: does anything work? Current hematologic malignancy reports. 2012; 7: 78-86.
- Marchioli R, Finazzi G, Landolfi R, Kutti J, Gisslinger H, Patrono C, et al. Vascular and neoplastic risk in a large cohort of patients with polycythemia vera. J Clin Oncol. 2005; 23: 2224-2232.
- Geyer H, Mesa RA. Assessing disease burden in patients with classic MPNs. Best Pract Res Clin Haematol. 2014; 27: 107-119.
- Johansson P, Mesa R, Scherber R, Abelsson J, Samuelsson J, Birgegard G, et al. Association between quality of life and clinical parameters in patients with myeloproliferative neoplasms. Leuk Lymphoma. 2012; 53: 441-444.
- Mesa RA, Niblack J, Wadleigh M, Verstovsek S, Camoriano J, Barnes S, et al. The burden of fatigue and quality of life in Myeloproliferative Disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007; 109: 68-76.
- Harrison CN, Koschmieder S, Foltz L, Guglielmelli P, Flindt T, Koehler M, et al. The impact of Myeloproliferative Neoplasms (MPNs) on patient quality of life and productivity: results from the international MPN Landmark survey. Annals of hematology. 2017; 96: 1653-1665.
- Anderson LA, James G, Duncombe AS, Mesa R, Scherber R, Dueck AC, et al. Myeloproliferative neoplasm patient symptom burden and quality of life: evidence of significant impairment compared to controls. Am J Hematol. 2015; 90: 864-870.
- Abelsson J, Andreasson B, Samuelsson J, Hultcrantz M, Ejerblad E, Johansson B, et al. Patients with polycythemia vera have worst impairment of quality of life among patients with newly diagnosed myeloproliferative neoplasms. Leuk Lymphoma. 2013; 54: 2226-2230.
- Scherber R, Dueck AC, Johansson P, Barbui T, Barosi G, Vannucchi AM, et al. The Myeloproliferative Neoplasm Symptom Assessment Form (MPN-SAF): international prospective validation and reliability trial in 402 patients. Blood. 2011; 118: 401-408.
- Emanuel RM, Dueck AC, Geyer HL, Kiladjian JJ, Slot S, Zweegman S, et al. Myeloproliferative Neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012; 30: 4098-4103.
- Vardiman JW. The World Health Organization (WHO) classification of tumors of the hematopoietic and lymphoid tissues: an overview with emphasis on the myeloid neoplasms. Chem Biol Interact. 2010; 184: 16-20.
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severityin cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999; 85: 1186-1196.
- Peruzza S, Sergi G, Vianello A, Pisent C, Tiozzo F, Manzan A, et al. Chronic Obstructive Pulmonary Disease (COPD) in elderly subjects: impact on functional status and quality of life. Respiratory medicine. 2003; 97: 612-617.