
Research Article
Ann Hematol Oncol. 2019; 6(9): 1266.
Clinical Analysis of the Risk Factors of Early and Late Phase Recurrence after Surgical Excision of Hepatocellular Carcinoma: Experience of One Center with 345 Patients
Tailai A1, Tianxing D1, Zhenyu Y2, Wei L3, Yingcai Z1 and Guoying W1,3*
1Department of Hepatic Surgery and Liver Transplantation Center, The Third Affiliated Hospital of Sun Yat-Sen University, China
2Organ Transplantation Institute, The Third Affiliated Hospital of Sun Yat-sen University, China
3Guangdong Key Laboratory of Liver Disease Research, The Third Affiliated Hospital of Sun Yat-sen University, China
*Corresponding author: Wang Guoying, Department of Hepatic Surgery and Liver Transplantation Center, The Third Affiliated Hospital of Sun Yat-Sen University, China
Received: June 22, 2019; Accepted: July 18, 2019; Published: July 25, 2019
Abstract
Background: Resection excision operation is an important treatment alternative for respectable HCC (hepatocellular carcinoma); unfortunately, many patients undergo the experience of early or late phase recurrence after surgical excision of the tumor lesion. Our study here aims to investigate the risk factors associated with early and late phase recurrence after surgery as well as to establish a predictive model to facilitate improving our predictive ability about HCC recurrence and accumulating the comprehensive treatment experience.
Methods: We conducted a retrospective analysis of 345 patients who received surgical excision of the tumor lesions in our hospital. Patients were divided into three classes: no recurrence, early phase recurrence and late phase recurrence. Risk factors of early and late recurrence were analyzed statistically. On the basis of the risk factors associated with early and late phase recurrence, the mathematics models for early and late phase recurrence were established.
Results: The cumulative survival rates without recurrence at 1,2,3,4, and 5 years were 68.8%, 40.1%, 35.7%, 30.2%, and 27.5% respectively. According to the widely accepted definition: early phase recurrence occurs within 2 years after the surgery and late phase recurrence happens 2 years after the resection. 197 patients had early phase recurrence and 25 ones had late phase recurrence. Cox multivariate proportion hazard model suggested that multiplicity of tumor lesions, high preoperative serum fibrinogen level, high preoperative serum GGT level and too much blood transfused are independent risk factors significantly correlated with early phase recurrence. In contrast with the risk factors associated with early phase recurrence, multiplicity of tumor lesions, severe liver cirrhosis and portal vein hypertension, high preoperative serum CHE level and high preoperative serum GGT level were identified as risk factors associated with late phase recurrence. Patients with at least two of the four early phase recurrence risk factors were much more likely to have early phase recurrence and patients with three or more late recurrence risk factors were prone to experience late phase recurrence.
Conclusions: Different kinds of risk factors are associated with early and late phase recurrence. Early phase recurrence occurs due to metastases within the liver while late phase recurrence happens as a result of impaired liver function reserve and carcinogenesis de novo from the cirrhotic liver.
Keywords: Hepatocellular carcinoma; Early and late phase recurrence; Surgical excision; Risk factors; Prognosis; High-risk group and low-risk group; Cut-off value
Introduction
As a common kind of malignant tumor, HCC (hepatocellular carcinoma) causes about 1 million deaths due to its increasing incidence annually and poor 5-year survival rate of less than 5% without treatment [1-4]. Surgery, which includes keratectomy and liver transplantation, is still believed by most surgeons to be the most effective treatment alternative for the patients with respectable HCC lesions. Although liver transplantation is the most curative treatment, it also has its own shortcomings. The lack of donor livers, long waiting time, higher perioperative morbidity and long-term or even lifetime immunosuppression therapy still restrict the wide application of the liver transplantation in the treatment of HCC. Over the last several decades surgical techniques and perioperative management of patients undergoing keratectomy have become more and more sophisticated which makes the procedure of resection of HCC much safer than that of several decades ago [1,3,4,5]. The safety of the surgical procedure is indicated by much lower mortality and morbidity rates than those of several decades ago [1,3,4,5]. Even after a safe surgical resection of the HCC, the long-term prognosis of patients with HCC remains poor due to the high incidence of recurrence (68%-96%) [1,4,5,7,9]. Thus it is critical to develop effective therapeutic methods to control tumor recurrence with an ultimate goal of prolonging the life of HCC patients. By now, various kinds of risk factors associated with HCC recurrence have been reported. These factors include tumor-related ones, background liver status, the type of the surgery and even some molecular and immunological markers [1-8,12,14,17,20]. However, the exact causes and mechanism of recurrence still remain mysterious. In this study, we investigated the pattern of recurrence time and a few potential risk factors that may help us predict the early and late phase recurrence of HCC after a hepatectomy for HCC.
Methods
Patients
Between March 2005 and May 2013, 475 patients received surgical excision of HCC at the department of hepatobiliary surgery and liver transplantation of The Third Affiliated Hospital of Sun Yat Sen University. Of all these patients, 130 were excluded from the study. 57 patients received other treatment options prior to the resection surgery and 73 ones had simultaneous intraoperative microwave ablation because of the multiple lesions within the liver. 345 patients were included in the present study. Curative resection includes the complete removal of the tumor and the visible portal vein tumor thrombus with a negative microscopic margin. Patients receiving anti-HBV before and after the hepatectomy are recorded in the study and we analyzed the relationship between the anti-HBV therapy and late phase recurrence. The procedures and the related methods of this study and using human sample had received approval by the Ethics Committee of our hospital before the implementation and all the patients involved in this research had signed written informed consent.
Surgical modalities
If 3 or more segments (according to the Coined classification were resected, the procedure was called a major hepatectomy. 180 (52%) patients underwent major hepatectomies, of whom 27 (5%) were resected 3 or more discontinuous segments. The resection operation of 165 patients were minor ones that include non-anatomical wedge resections (no more than two segments) or enucleations (50, 14.3%) and left lateral segmentectomy (44, 12.8%). The average number of resected hepatic segments is 3.2 0.4 (range 0-6). Anatomical resection, defined as any type of systematic resection of the portal region based on the Coined classification system, was performed in 299 patients (75.1%), while 99 patients underwent non-anatomical resections (24.9%. In this study we classified incomplete removal of tumor-bearing portal region such as wedge resection or enucleation as non-anatomical resection while discontinuous segments resection was defined as anatomical resection as long as each resection of the patient was anatomical resection.
Diagnosis of HCC
Nowadays most HCC patients were detected and evaluated using contrast ultrasonography, contrast CT and MRI. If the lesion is larger than 2cm in diameter, a single imaging modality with arterial hypervascular and venous washout characteristic is suggestive of HCC; otherwise 2 or more kinds of imaging modalities with arterial hypervascular and venous washout characteristic are needed to confirm the diagnosis of HCC. The diagnosis of HCC patients were confirmed by histopathological investigation after the resection. Pathological grading was based on the Edmodson-Steiner criteria.
Follow-up
All the follow-up processes of the patients after resection were carried out in the outpatient or inpatient department of our hospital and the recurrence of HCC was closely monitored prospectively. The follow-up protocol was made up of monthly serum AFP (alpha-fetoprotein) monitoring and contrast ultrasonography, contrast CT or MRI once every 3 months after the resection of the HCC lesion. The changes of the serum tumor markers before and after the surgical operation as well as those at the confirmation of the recurrence were assessed. Tumor recurrence was confirmed according to the same criteria applied to the initial diagnosis of HCC and if hepatic re-resection was done, the recurrence was diagnosed by histopathological investigation. The number, size and location of recurrence (intrahepatic or extrahepatic) were then recorded. Recurrences outside of the liver (i.e metastases) were investigated by contrast ultrasonography, CT, MRI or PET-CT using 18F-FDG.
Statistical analysis
Descriptive statistics had several parameters including mean, range, standard deviation and proportion. For the continuous variables that have been previously widely used by the clinicians, the widely accepted cut-off values of these variables were directly used, otherwise the cut-off values were calculated by the ROC curve method. In univariate analysis, χ2 test was adopted to determine the variables significantly associated with early and late phase recurrence. The multivariate analysis of prognostic factors for HCC early and late phase recurrence was done using the Cox’s proportional hazards model. All the 23 variables were entered into a backward stepwise regression model. Step selections were based on the maximal likelihood ratio tests and only significant variables were reserved in the multivariate Cox’s proportional hazards model analysis. The Kaplan-Meir method was used to evaluate survival rates and the logrank test was applied to compare survival rates. SPSS18.0 for Windows (Chicago IL, USA) was used to to perform all the statistical evaluation. For ROC (receiver operating characteristic) curve analysis, we used the Medcalc (version120 to calculate the sensitivity, specificity, area under the curve and to select the optimal cut-off value for predicting HCC recurrence. A variable was considered statistically significant when its P value was lower than 0.05.
Results
Patient characteristics
306 men (88.7%) and 39 women (11.3%) were incorporated in the present study with a mean age of 50.01±11.62 years. Assessed by the Child-Pugh classification system, all the patients belonged to grade A or B. 317 patients (92.2%) belonged to Child-Pugh A and 28 patients (7.8%) belonged to Child-Pugh B. At the start of the hepatectomy, 237 patients (68.7%) had only one tumor lesion and 108 patients (31.3%) had multiple tumors. The median nodule diameter was 5.38 cm (range 1.0-18.2cm). Regarding the etiology of the HCC, 310 (77.9%) of all the patients were HBV-positive while only 3 were confirmed HCV-positive. All the patients had liver fibrosis of different grades and 272 patients (68.3%) had cirrhosis background. Table 1 shows the demographics of all the patients that contain preoperative, intraoperative and tumor-related parameters pertain to the initial hepatectomy.
Variables
Mean±SD(range)/(n,%)
Gender
Male
306(88.7)
Female
39(11.3)
Age(years)
50.01±11.62(16-79)
Smoking
No
229(66.4)
Yes
116(33.6)
Type II Diabetes Mellitus
No
311(90.1)
Yes
34(9.9)
Preoperative antiviral therapy
No
180(52.2)
Yes
165(47.8)
Portal hypertension and cirrhosis
No
108(31.3)
Mild to moderate
226(65.5)
Severe
11(3.2)
Maximal diameter of the tumor
5.80±3.58(1.00-18.20)
Number of the tumor lesions
Single
237(68.7)
Multiple
108(31.3)
Vascular invasion
No
200(58.0)
Yes
145(42.0)
Extrahepatic metastasis
No
336(97.4)
Yes
9(2.6)
Cancerous thrombus of the IVC or portal vein
No
307(89.0)
Yes
38(11.0)
Rupture of the tumor
No
323(93.6)
Yes
22(6.4)
Margin
Negative
339(98.3)
Positive
6(1.7)
AFP
<100
172(49.9)
101-200
34(9.9)
201-400
23(6.7)
>400
116(33.6)
Variables
Mean±SD(range)/(n,%)
Neutrophil cell count
28.34±21.58(1.0-113.0)
Platelet cell count
178.49±78.63(2.41-465.00)
Lymphocyte count
1.67±0.66(0.23-4.21)
Mononuclear cell count
0.52±0.46(0.04-6.25)
ALP
89.47±40.88(32-305)
ALT
49.76±55.22(6-562)
AST
49.84±48.35(12-477)
TC
4.46±1.10(1.37-8.15)
CHE
6742.32±3055.07(1711-49262)
ALB
39.77±4.21(22.4-52.5)
PA
176.63±58.19(24-357)
GGT
99.65±136.12(14-1549)
ADA
13.93±6.05(1-43)
FIB
3.27±1.19(1.17-10.27)
Child-Pugh Grading
A
317(92.2)
B
27(7.8)
Intraoperative blood transfusion
415.22±651.04(0-4200)
AST/ALT
1.20±0.67(0.34-4.70)
Pathological differentiation
Well
59(17.1)
Moderate
261(75.7)
Poor
25(7.2)
Recurrence
No
123(35.7)
Yes
222(64.3)
Follow-up time (months)
28.34±21.58(1-113)
Follow-up time without recurrence (months)
18.02±19.22(0.5-108)
AFP: Alpha Fetoprotein; ALP: Alkaline Phosphatase; ALT: Alanine Aminotransferase; AST: Aspartate Aminotranferase; TC: Total Cholesterol; CHE: Cholinesterase; ALB: Albumin; PA: Pre-Albumin; GGT: Glutamyl Transpeptidase; ADA: 3.5.4.4 Adenosine Deaminase; FIB: Fibrinogen.
Table 1: The descriptive analysis of the clinic pathological factors of the 345 patients (N = 345).
Cumulative risk for recurrence
237 patients underwent the experience of recurrence during the follow-up time of 1 to 113 months after surgery. And the mean time from surgery to tumor recurrence was 18.02±19.22 months (range 0.5 to 108 months). Of these patients who had recurrence, 210 (89%) patients experienced intrahepatic recurrence while 21 (9%) had intrahepatic and extrahepatic recurrence at the same time or had extrahepatic recurrence subsequently. The remaining 6 (2.0%) patients had extrahepatic tumor recurrence without any intrahepatic recurrence lesions. Extrahepatic metastases were diagnosed through imaging techniques and (or) pathological analysis of the resected extrahepatic recurrence lesions. The sites where the extrahepatic tumor recurrence occurred included the lungs (18 patients), brain (5 patients), bone (3 patients) and extrahepatic bile duct (1 patients). There was no recurrence occurring near the cut surface. Almost all the recurrences were multiple in number and occurred in both liver lobes or opposite lobe. One hundred and eight patients did not have the experience of recurrence after a mean follow-up time of 31.5 months. The cumulative recurrence-free Kaplan-Meier curve of the 345 patients having received hepatectomy surgery was demonstrated in (Figure 1). The cumulative recurrence-free survival rate was 68.8%, 40.1%, 35.7%, 30.2% and 27.5% at 1,2,3,4 and 5 years respectively. The recurrence time distribution curve of the 237 patients with recurrence was shown in (Figure 2). Two distinctively different recurrence peaks were detected according to the recurrence time distribution curve. The first peak was at one year after the surgery and it was the most likely time when recurrences occurred. The second peak was at 4 years after the hepatectomy. We could also infer from the recurrence time distribution pattern that recurrence after hepatectomy should be classified into early phase (within 2 years) and late phase recurrence (after two years).
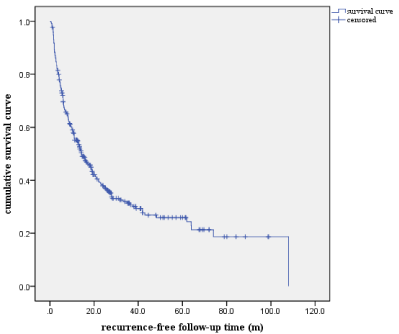
Figure 1: The cumulative recurrence-free survival curve of the 345 patients.
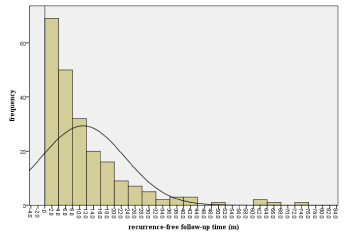
Figure 2: The distribution of the time from the hepatectomy to recurrence of
the 237 patients.
Univariate analysis of early and late phase tumor recurrence
197 patients experienced early phase recurrence, while late phase recurrence happened to forty patients. The results of risk factors associated with early phase recurrence and late phase recurrence by the means of univariate analysis were shown in (Table 2) and (Table 4). Fourteen factors significantly associated with early phase tumor recurrence were determined through univariate analysis, namely pathological differentiation, number of the tumor lesions, vascular invasion, neutrophil cell count, AST, ALB, PA, GGT, FIB, Intraoperative blood transfusion, ALT, CHE, ADA and maximal diameter of the tumor. It was also through univariate analysis that eleven parameters significantly associated with late phase tumor recurrence were identified, i.e portal hypertension and cirrhosis, maximal diameter of the tumor, platelet cell count, lymphocyte count, TC, ALB, CHE, GGT, ADA, FIB and Child-Pugh grading.
Variables
No recurrence
Recurrence
Total
χ2value
P value
Gender
Male
94
142
236
2.053
0.152
Female
17
15
32
Smoking
Yes
34
57
91
0.934
0.334
No
77
100
177
Type II Diabetes Mellitus
Yes
7
19
26
2.493
0.114
No
104
138
242
Preoperative antiviral therapy
Yes
51
83
134
1.246
0.264
No
60
74
134
Portal hypertension and cirrhosis
No
41
47
88
2.987
0.07
Mild to moderate
70
104
174
Severe
0
6
6
Vascular invasion
Yes
32
59
91
2.221
0.01*
No
79
98
177
Extrahepatic metastasis
Yes
2
2
4
0.123
0.726
No
109
155
264
AFP
Variables
No recurrence
Recurrence
Total
χ2 value
P value
1-100
57
83
140
1.126
0.771
101-200
13
17
30
201-400
9
8
17
>400
32
49
81
Child-Pugh grading
A
107
43
250
2.930
0.0087
B
4
14
18
Pathological differentiation
Well
28
18
46
9.422
0.01*
Moderate
79
128
207
Poor
4
11
15
Maximal diameter of the tumor
=3cm
68
59
127
14.627
0.001*
<3cm
43
98
141
Neutrophil cell count
=3.72
78
94
192
3.058
0.08
>3.72
33
63
96
PA
=195
77
90
167
21.16
<0.001*
>195
30
71
101
GGT
=96
99
107
206
16.182
<0.001*
>96
12
50
62
Age
=60
78
121
199
1.573
0.21
>60
33
36
69
Number of tumor
Lesions
>3
95
111
206
19.951
<0.001*
=3
16
46
62
Platelet cell count
=150
36
70
106
4.109
0.045
Variables
No recurrence
Recurrence
Total
χ2 value
P value
>150
75
87
162
Lymphocyte count
=1.72
53
106
158
11.480
0.006*
>1.72
59
51
110
Mononuclear cell count
=0.57
89
111
200
3.086
0.079
>0.57
22
46
68
ALP
=83
74
81
161
3.433
0.064
<83
37
70
107
ALT
=52
27
17
44
8.631
0.003*
>52
84
140
224
Table 2: Univariate analysis of the risk factors associated with early phase recurrence.
AST
=38
76
78
154
9.390
0.002*
<38
35
79
114
TC
=4.1
50
59
109
1.465
0.326
>4.1
58
88
146
CHE
=7556
60
115
175
10.573
0.001*
>7556
51
42
93
ALB
=39.2
71
77
148
5.854
0.016*
>39.2
40
80
120
GGT
=96
99
127
206
16.182
<0.001
>96
12
50
62
ADA
=19
99
127
206
4.388
0.048*
>19
12
30
42
FIB
Variables
No recurrence
recurrence
Total
χ2 value
P value
=2.48
93
109
202
7.221
0.007*
<2.48
18
48
66
AST/ALT
=0.9
52
65
117
0.765
0.382
>0.9
53
78
131
Intraoperative blood transfusion
=400
86
96
182
7.985
0.005*
>400
25
61
86
Table 2 of 1:
Multivariate analysis of the independent adverse risk factors significantly correlated with early phase and late phase recurrence were done by Cox’s multivariate proportional hazard model analysis. Contrary to univariate analysis, only 4 factors were proven to be associated with early phase tumor recurrence: Number of tumor lesions [OR=2.139 P ‹0.001 95% CI: 1.535-2.982] FIB [OR=1.604, P =0.01, 95% CI: 1.121-2.294 ], GGT [OR=2.091, P ‹0.001, 95% CI: 1.478-2.958] and Intraoperative blood transfusion [OR=1.611, P =0.005, 95% CI: 1.158-2.241 ]. Otherwise, 4 factors were detected to induce late phase tumor recurrence, namely Number of tumor lesions [OR=3.895, P =0.003, 95% CI: 1.580-9.604], Portal hypertension and cirrhosis [OR=3.762, P =0.003, 95% CI: 1.677-8.485], CHE [OR=1.000, P =0.007, 95% CI: 0.999-1.004] and GGT [OR=1.004, P =0.030, 95% CI: 1.000-1.008]. The results of Cox’s multivariate proportional hazard model analysis of early and late phase recurrence are shown in (Table 3) and (Table 5).
Variables
β
SE
Wald
P
95%CI
OR
Number of tumor
Lesions
0.760
0.169
20.131
0.000*
1.535-2.982
2.139
FIB
0.473
0.183
6.696
0.010*
1.121-2.294
1.604
GGT
0.738
0.177
17.365
0.000*
1.478-2.958
2.091
Intraoperative blood transfusion
0.477
0.168
8.027
0.005*
1.158-2.241
1.611
Table 3: Cox’s multivariate proportional hazard model analysis of early phase recurrence.
Variables
No recurrence
Recurrence
Total
χ2 value
P value
Gender
Male
104
23
127
0.946
0.530
Female
19
2
21
Age
Variables
No recurrence
Recurrence
Total
χ2 value
P value
=60
35
6
41
0.206
0.650
<60
88
19
107
Smoking
No
89
18
107
0.001
0.971
Yes
34
7
41
Type II Diabetes Mellitus
No
112
24
136
0.681
0.691
Yes
11
1
12
Preoperative antiviral therapy
No
64
15
79
0.530
0.467
Yes
59
10
69
Portal hypertension and cirrhosis
No
43
6
49
6.094
0.048*
Mild to moderate
79
17
96
severe
1
2
3
Maximal diameter of the tumor
=9.5cm
119
21
140
6.603
0.028*
>9.5cm
4
4
8
Number of tumor
lesions
=3
118
22
40
2.558
0.120
>3
5
3
8
Vascular invasion
No
81
18
99
0.354
0.552
Yes
42
7
49
Extrahepatic metastasis
No
121
25
146
0.412
0.521
Yes
2
0
2
Table 4: Univariate analysis of the risk factors associated with late phase recurrence.
Cancerous embolism
Variables
No recurrence
Recurrence
Total
χ2 value
P value
No
118
22
140
1.719
0.352
Yes
5
3
8
Margin
Negative
121
23
144
3.210
0.073
Positive
2
2
4
AFP
1-100
62
14
76
0.965
0.810
101-200
14
2
16
201-400
10
3
13
>400
37
6
43
Neutrophil cell count
=2.59
36
12
48
3.327
0.068
>2.59
87
13
100
Platelet cell count
=180
61
20
81
7.753
0.005*
>180
62
5
67
Lymphocyte count
=1.51
42
15
57
5.865
0.015*
>1.51
81
10
91
Mononuclear cell count
=0.28
20
8
28
3.356
0.067
>0.28
103
17
20
ALP
=54
23
2
25
1.694
0.193
>54
100
23
123
ALT
=45
92
15
107
1.271
0.132
>45
31
10
41
AST
=32
60
8
68
2.356
0.125
>32
63
17
80
Table 4of 1:
Variables
No recurrence
Recurrence
Total
χ2 value
P value
TC
=4.3
66
7
73
4.833
0.028*
>4.3
53
16
69
ALB
=41.9
78
21
99
3.976
0.046*
>41.9
45
4
49
CHE
=7403
66
21
87
7.895
0.007*
>7403
57
4
61
PA
=195
62
16
78
1.918
0.186
>195
59
8
67
GGT
=58
72
7
79
7.785
0.008*
>58
51
18
69
ADA
=22
115
20
135
4.723
0.03*
>22
8
5
13
FIB
=2.19
107
17
124
5.516
0.019*
<2.19
16
8
24
Intraoperative blood transfusion
=1550ml
7
1
8
1.493
0.222
<1550ml
116
25
140
AST/ALT
=0.77
32
10
42
1.999
0.151
>0.77
91
15
106
Child-Pugh grading
A
117
20
137
8.525
0.014*
B
5
6
11
Pathological differentiation
Variables
No recurrence
Recurrence
Total
χ2 value
P value
Well
28
11
39
4.829
0.089
Moderate
88
13
101
Poor
7
1
8
Table 4 of 2:
Variables
β
SE
Wald
P
OR
95%CI
Number of tumor
Lesions
1.360
0.460
8.723
0.003
3.895
1.580-9.604
Portal hypertension and cirrhosis
-2.869
1.005
10.475
0.003
3.762
1.677-8.485
CHE
0.049
0.000
7.368
0.007
1.000
.999-1.004
GGT
0.08
0.002
4.717
0.030
1.004
1.000-1.008
Table 5: Cox’s multivariate proportional hazard model analysis of late phase recurrence.
Predicting early phase tumor recurrence
By the means of ROC analysis, the cut-off value that best predicts the risk of early phase recurrence is 2. The patients in our research were then stratified into high-risk (patients with at least 2 adverse independent risk factors) and low-risk (absence of any or only 1 adverse independent risk factors) groups. Figure 3 shows the cumulative recurrence rates for the high-risk and low-risk groups. High-risk patients and low-risk patients were proven to have significantly different early phase recurrence rates (P‹0.001, log-rank test).
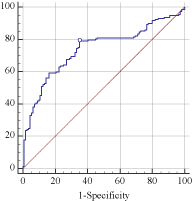
Figure 3: The ROC curve for the prediction of the combination of all the
independent risk factors for early phase recurrence (AUC=0.739, P‹0.001).
Establishing and validation of the mathematics model for early phase tumor recurrence
Cox’s multivariate proportional hazard model analysis identified that serum GGT level, tumor lesion number, preoperative fibrinogen level and intraoperative blood transfusion are the independent risk factors for early phase tumor recurrence. On the basis of the results of Cox’s multivariate proportional hazard model analysis, the mathematics model for early phase tumor recurrence wash (t)=h0(t) exp(0.738*GGT+0.760*tumor lesion number+0.473*preoperative fibrinogen level+0.477*intraoperative blood transfusion).
Predicting late phase tumor recurrence
By the means of ROC analysis, the cut-off value that best predicts the risk of late phase recurrence is 3. The patients in our research were then stratified into high-risk (patients with at least 3 adverse independent risk factors) and low-risk (less than 3 adverse independent risk factors) groups. Figure 3 and Figure 5 shows the cumulative recurrence rates for the high-risk and low-risk groups. High-risk patients and low-risk patients were proven to have significantly different late phase recurrence rates (P‹0.001, log-rank test) (Figure 6).
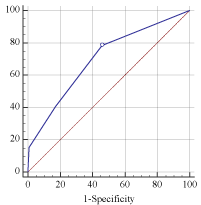
Figure 4: The ROC curve determining the best cut-off value predicting early
phase recurrence (AUC=0.700, P‹0.001).

Figure 5: Cumulative recurrence-free survival curve for early phase
recurrence after hepatectomy (patients were stratified into high-risk and lowrisk
group) (log-rank test, χ2=32.86, P‹0.001).
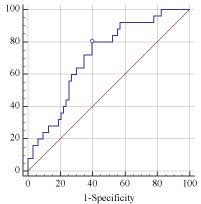
Figure 6: The ROC curve for the prediction of the combination of all the
independent risk factors for late phase recurrence (AUC=0.709, P‹0.001).
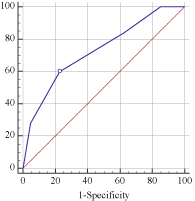
Figure 7: The ROC curve determining the best cut-off value predicting late
phase recurrence (AUC=0.728, P‹0.001).
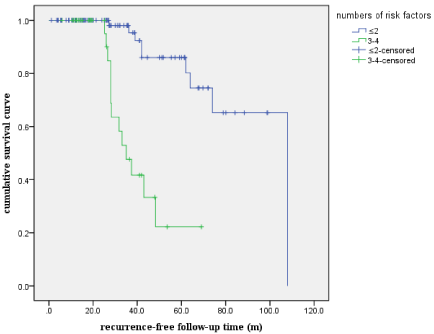
Figure 8: Cumulative recurrence-free survival curve for early phase
recurrence (Patients were divided into high-risk and low-risk group) (log-rank
test, χ2 value = 33.57, P‹0.001).
Establishing the mathematics model for late phase tumor recurrence
Cox’s multivariate proportional hazard model analysis identified that serum GGT level, tumor lesion number, preoperative CHE level and background liver cirrhosis status were the independent risk factors for late phase recurrence. On the basis of the results of Cox’s multivariate proportional hazard model analysis, the mathematics model for late phase tumor recurrence wash (t)=h0(t)exp(1.360 *tumor lesion number-2.869*background liver cirrhosis status +0.049*CHE+0.08*GGT).
Discussion
Hepatectomy can simultaneously remove the main tumor lesion and the surrounding tissue that may contain microscopically lesions that cannot be detected by conventional imaging techniques. And hepatectomy can be carried out almost without any waiting time. The two advantages mentioned above make hepatectomy the first choice treatment for most respectable HCC. Although the prognosis for most HCC patients has improved a lot over the last several decades, tumor recurrence remains the most common cause of treatment failure and even the deaths of HCC patients [1,2,6,9,17,20,22]. It has been reported in several studies that early phase recurrences are mainly due to intrahepatic dissemination and therefore are associated with aggressive pathological tumor factors such as vascular invasion, tumor diameter, multiplicity of tumor lesions, incomplete tumor capsule and tumor cell dissemination during the surgery [6,9,10,21,27,30,38]. It has been postulated that the factors mentioned above are very much likely to lead to intrahepatic metastasis and subsequently to early phase tumor recurrences [2,5,9,12,19,24,46]. Many experts believe early phase recurrences are associated with invasive nature of the tumor cells [1-5,15,26,34,42,46]. However, some other researchers found that liver status plays a much more important role in the tumor recurrence [5,12,26,34,45]. Thus, the precise mechanism of tumor recurrence still remains controversial.
In our study, we divided the tumor recurrences into two categories according to the recurrence time distribution pattern. Recurrences within two years after the surgery were defined as early phase recurrences and recurrences beyond two years after the surgery were defined as late phase recurrences. Moreover, it has been suggested by many experts in HCC treatment that different phases of recurrences are associated with different kinds of risk factors [1,7,9,18,25,39,42,46]. These findings may in part explain why many previous studies came to conflicting conclusions, since these studies did not distinguish early phase recurrences from late phase recurrences. Poon et al, were the first to distinguish early recurrences from late phase recurrences and find different phases of recurrences were correlated with different kinds of risk factors [2,9,14,20,27,36,42,46]. So they furthermore suggested that early phase recurrences mainly originated from intrahepatic metastases and late phase recurrences actually represented new tumor formation. However, the exact time that separates early phase recurrences from late phase recurrences is still controversial. Poon et al, suggested one year, which is different from our the two years we concluded from the recurrence time distribution pattern [2,9,14,20,27,36,42,46]. This difference might be due to different patient features. Imaura et al, also proposed that two years should be the demarcation time, although their research determined different risk factors from ours [5,14,17,18,23,46]. (The HCC patients in their research are HCVpositive, whereas most patients in our research are HBV-positive).
Our research found 4 risk factors associated with early phase recurrence, namely tumor lesion number, serum GGT level, high preoperative fibrinogen level and intraoperative blood transfusion. The identification of portal vein and IVC (inferior vena cava) cancerous thrombus as an adverse independent risk factors indicates that the main route by which early phase recurrence occurs is spreading through the portal vein. Several previous researches have pointed out that preoperative vascular invasion or even portal vein and IVC cancerous thrombus often heralds fulminant recurrence and extremely terrible prognosis [1-4,7,14,27,33,46].
Tumor lesion multiplicity is another adverse risk factor associated with early phase tumor recurrence and the possible explanation is that tumor lesion multiplicity reflects microscopic vascular invasion. Some other studies reported that serum AFP level, pTNM score, histological differentiation and tumor diameter are useful prognostic factors to predict early phase tumor recurrence [1- 6,10,19,21,37,44,46]. However, in our research, none of the factors mentioned above were proven to be significantly associated with early phase tumor recurrence. Contrary to the results of other studies, none of the surgery-related factors were identified to contribute to early phase recurrence. To sum up, the vascular invasion behavior of tumor cells is believed to be the most important mechanism how the early phase tumor recurrence occurs.
Besides the factors that reflect tumor cell vascular invasion nature, some other factors which include serum GGT level, preoperative fibrinogen level and intraoperative blood transfusion are also identified to be independent adverse risk factors of early phase tumor recurrence. The following three passages will be dedicated to discussing how these three factors are related to early phase tumor recurrence.
GGT, also known as gama glutamyl transpeptidase, is widely distributed through the human body tissues. The kidney tissue has the most abundant GGT content, followed by pancreas, liver and heart tissue [5,11,22,36,44]. Elevated serum GGT level is most often seen in hepatobiliary diseases. In HCC patients, serum GGT level is often several times above the upper limit. In our study, GGT is proven to be negatively related to early phase tumor recurrence. The GGT gene hypomethylation status of the HCC malignancy is the possible reason why GGT level is significantly elevated in most HCC patients [24,30,44]. Both the malignant cells in the tumor and adjacent inflamed liver tissue are responsible for the significantly elevated serum GGT level [24,30,41,44]. So significantly elevated serum GGT level can in part reflect the invasive nature of the tumor cells of HCC patients. So we can also relate elevated serum GGT level to the early phase tumor recurrence after surgical removal of all the visible HCC lesions. GGT has different isoforms which include F(fetus) type, P (placenta) type and H (HCC malignant cell ) type [44]. Of all these three types of GGT, HS-GGT is most valuable in the diagnosis of HCC [44]. Apart from aiding in the diagnosis of HCC, GGT especially HSGGT can be used to predicting tumor recurrence [44].
As one of the most important technologies promoting the development of surgical treatment, blood transfusion plays an irreplaceable role in the treatment of HCC. Opelz et al, found that blood transfusion before kidney transplantation could prolong the graft survival time through suppression of the immune system of the recipients [21,32,35]. Subsequent researches concluded that patients receiving blood transfusion especially massive blood transfusion were much more likely to experience tumor recurrence and to have a worse prognosis (breast cancer lung cancer and renal cell carcinoma and so on) [21,32,35]. Despite the discoveries mentioned above, the precise mechanism of this phenomenon still remains unknown. The following studies revealed that blood transfusion inhibits the immune system of the patients, such as the reactivity of the lymphocytes, NK cells (natural killer) and macrophages [21,32,35]. Blood transfusion makes the system produce all kinds of inhibitory factors enabling the tumor cells to escape from the attacks of the immune system and therefore to maintain able to grow and spread to other sites of the body. The fact that massive blood transfusion promotes tumor growth and recurrence also applies to HCC. Many retrospective studies of independent risk factors associated with HCC tumor growth and recurrence found that massive blood transfusion were significantly adversely correlated with recurrence and worse prognosis of HCC [21,32,35]. The development of modern surgical instruments, the perioperative management and the comprehensive treatment of HCC patients make the prognosis of the HCC patients much better than that of several decades ago [1-6,14,21,37,46]. However, the prognosis of HCC patients remains relatively poorer than other malignant diseases. And tumor recurrence remains the main factor affecting the long-term survival rates of HCC patients [1-9,15,26,33,46]. Intraoperative massive blood transfusion increases the recurrence rate and adversely affected the long-term prognosis of HCC patients. Hepatobiliary surgeons must do their best to improve their surgical skills and make use of the modern surgical instruments to reduce the unnecessary or uncalled-for blood transfusion to improve the prognosis of HCC patients to the maximum extend.
Many kinds of malignant tumors are with elevated serum fibrinogen and elevated level of fibrinogen is associated with tumor recurrence, vascular invasion and metastasis [6,14,25,37,44]. HCC is not exceptional. The liver plays a vital role in maintaining the normal function of the blood coagulation system. First, the liver syntheses many kinds of coagulation, anticoagulation and fibrinolytic factors. Second, the liver is responsible for eliminating the activated coagulation factors, plasminogen activator and fibrinogen degradation products. So, the liver is vital in keeping the balance between the coagulation system and the anticoagulation system. The coagulation state of the HCC patients is varied among the patients while most patients who have malignant tumors are usually in hypercoagulative state. The reason for the phenomenon mentioned above is that HCC patients usually have cirrhosis background. Some patients are in hypercoagulative state because they have multiple tumor lesions or lesions invading the vascular system which produce too much procoagulation factors while the background cirrhosis is not so severe. Some other patients are in hypo coagulation state because of the too severe cirrhosis background. As one of the factors involved in the coagulation system, fibrinogen level shows the same pattern as the hypercaogulation state. Normally, patients with liver diseases usually has low levels of fibrinogen, since the ability to produce fibrinogen of the patients with liver diseases is compromised. So, the tumor stage and the liver function are two main factors affecting the levels of fibrinogen. Fan et al found that HCC HepG2 produced more fibrinogen than normal cells [4,8,13,22,47]. They also concluded that the levels of fibrinogen of the patients with vascular invasion or cancerous thrombus were much higher than those of the patients without vascular or cancerous thrombus [4,8,13,22,47]. That the level of fibrinogen is significantly positively associated with tumor stage was also confirmed [4,8,13,22,47].`The fibrinogen level of stage III and IV patients is significantly higher than that of stage I and II patients when the patients have the same liver function status [4,8,13,22,47]. Pajumbo et al, found mice without fibrinogen expression are immune to tumor recurrence and metastasis, which was consistent with many clinical researches [4,8,13,22,47]. Possible reasons why fibrinogen is associated with tumor recurrence and metastasis are as follows: (1) fibriogen molecule serves as a bridge of tumor cells, platelet cells and endothelial cells to facilitate the recurrence and metastasis of tumor cells, (2) fibrinogen molecule helps the positioning and attachment of the malignant microembolism in the targeted organ, which is vital to tumor recurrence and metastasis, (3) fibriogen molecules serve as the temporary mesenchymal tissue that provide the tumor cells with nutrients and oxygen, (4) the microcomplex of fibrinogen molecules, tumor cells and platelets protect the tumor cells from attacks of the immune system [4,8,13,22,47]. In summary, serum fibrinogen level can be used as one of the markers predicting the tumor development, local invasion and metastasis.
Contrary to early phase tumor recurrence, late phase tumor recurrence is associated with different risk factors. Tumor lesion number, background liver cirrhosis status, serum CHE level and GGT were found to be independent risk factors for HCC late phase recurrence. As discussed above, tumor lesion multiplicity is one risk factor for early phase recurrence, but we can also explain that tumor multiplicity implies that the liver is severely damaged and is prone to form new tumor lesions in the diseased tissue. It has been confirmed by many previous studies that late phase HCC recurrence after surgery is mainly due to intrahepatic carcinogenesis de novo and the microenvironment is the basis of tumor late phase recurrence [1-5,19,25,37,45,46]. Although liver cirrhosis status was found to be adversely affect tumor late phase recurrence, Li Bo et al, did not confirm liver cirrhosis as an independent risk factor for late phase tumor recurrence [46]. But their research concluded that continuous variables such as albumin level and ICGR15 (indocyanine green retention after 15 minutes) could more accurately reflect the damage degree of remnant liver tissue than categorical variables such as liver cirrhosis status [46]. ICGR15 test have been adopted by many surgeons and the hospital we work with has just initiated ICGR15 test. So we could have analyzed tumor late phase recurrence more accurately and objectively if we had adopted ICGR15 test to reflect liver function reserve instead of liver cirrhosis status. Although many surgeons believed antiviral therapy preoperatively and postoperatively may reduce the recurrence risk, we did not confirm this conclusion, however. It may be due to the fact that many patients lack the data of HBV infection and antiviral therapy. Li Bo et al, as well as many other surgeons concluded that perioperative antiviral therapy, no matter preoperatively or postoperatively, might reduce tumor late phase recurrence to a rather great extend [5,17,27,45,46]. We adopted HBVDNA to reflect HBV infection status and analyzed the relationship between preoperative antiviral therapy and tumor late phase recurrence. However, the result of our research was not so hopeful as other studies. Although we have not confirmed that perioperative antiviral therapy reduces the tumor recurrence risk, we still recommend that antiviral therapy be initiated perioperatively given many other experts confirmed the finding. Serum GGT level significantly increases either when tumor vascular invasion occurs or remnant liver damage is severe. We can explain why serum GGT level is significantly associated with tumor late recurrence since elevated GGT level could be related to cirrhotic liver background.
CHE, also known as cholinesterase, has two types, namely true CHE and false CHE. True CHE is mainly located within the neuromuscular junction and responsible for the breakdown of acetylcholine while false CHE is widely distributed all over the whole body (mainly the brain, serum, liver and intestine and kidney). The CHE in the serum is mainly produced by the liver. Most of the HCC patients have decreased CHE level, since most patients have the cirrhosis background (the liver produces most of the CHE in the serum). Our study found decreased CHE level was significantly associated with late phase tumor recurrence and the possible mechanisms are as follows. The liver produces most of the CHE in the serum and thus serum CHE level is one marker of cirrhosis and liver function reserve. Low serum concentration of CHE is indicative of liver microenvironment damage and thus is associated with late phase recurrence. Zhao et al found in their study that most of the molecules of the cholinergic system were expressed in HCC tumor tissue and the expression of CHE was significantly lower than the adjacent normal liver tissue or even the cirrhotic tissue [43]. It was also discovered in the experiment that CHE molecules could inhibit the growth and cloning of SMMC-7221 and HepG2 cells and Ache is the direct acting molecule of the inhibiting procedure [43]. They also confirmed that the possible molecular signal transduction pathway involved was inhibition of the MAPK-PI3K/Akt [43]. So the low serum CHE level is indicative of low expression of CHE in the tumor cells and cirrhotic tissue and thus is significantly associated with tumor late phase recurrence [43].
In summary, our study finds that intrahepatic metastasis from the primary tumor contributes to the early phase tumor recurrence after the surgery and the carcinogenesis de novo from the cirrhotic liver is the main reason why late phase tumor recurrence occurs. So it is understandable that early phase tumor is mainly associated with tumor-related factors and late phase tumor recurrence is chiefly associated with factors reflecting the severity of the damage of the remnant liver tissue. The recurrence and metastasis of the tumor can be avoided or delayed at least through the following means: to limit the extend of the excising and adopt sophisticated surgical skills to reduce the blood loss and the blood transfusion as far as possible, to adopt comprehensive treatment protocols for the patients with vascular invasion or cancerous thrombus, to control the longstanding infection of the HCC patients to preserve the liver function reserve of the remnant liver lobes. And furthermore, patients who are at high risk of recurrence, no matter early phase or late phase, should be closely monitored and receive corresponding treatment. Through all the efforts mentioned above, we can hope to increase the recurrence-free survival time of the patients as far as possible.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 81470870, 81570593), Sci-tech Research Development Program of Guangzhou city (No. 2014Y2-00200, 201604020001, 201508020262, 201400000001-3, 201607010024), National 13th Five-Year Science and Technology Plan Major Projects of China (2017ZX10203205-006-001); Guangdong Key Laboratory of Liver Disease Research (2017B030314027).
References
- Kim JH, Choi MS, Lee H. Clinical features and prognosis of hepatocellular carcinoma in young patients from a hepatitis B-endemic area. J Gastroenterol Hepatol. 2010; 21: 588-594.
- Lam VW, Ng KK, Chok KS. Risk factors and prognostic factors of local recurrence after radiofrequency ablation of hepatocellular carcinoma. J Am Coll Surg. 2011; 207: 20-29.
- Clark HP, Carson WF, Kavanagh PV. Staging and current treatment of hepatocellular carcinoma. Radiographics. 2009; 25: S3-23.
- Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2012; 4: 424-432.
- Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2013; 25: 181-200.
- Yi NJ, Suh KS, Kim T, Kim J. Current role of surgery in treatment of early stage hepatocellular carcinoma: resection versus liver transplantation. Oncology. 2013; 75: 124-128.
- Fan ST, Lo CM, Liu CL. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 2009; 229: 322-330.
- Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int. 2011; 7: 237-257.
- Poon RT, Fan ST, Lo CM. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002; 235: 373-382.
- Tsai TJ, Chau GY, Lui WY, Tsay SH, King KL, Loong CC, et al. Clinical significance of microscopic tumor venous invasion in patients with respectable hepatocellular carcinoma. Surgery. 2010; 127: 603-608.
- Cha C, Fong Y, Jarnagin WR, Blumgart LH, DeMatteo RP. Predictors and patterns of recurrence after resection of hepatocellular carcinoma. J Am Coll Surg. 2009; 197: 753-758.
- Nagano Y, Shimada H, Takeda K, Ueda M, Matsuo K, Tanaka K, et al. Predictive factors of microvascular invasion in patients with hepatocellular carcinoma larger than 5 cm. World J Surg. 2008; 32: 2218-2222.
- Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopatho-logical factors. Ann Surg Oncol. 2010; 15: 1375- 1382.
- Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 2005; 83: 1219-1222.
- Nagasue N, Uchida M, Makino Y, Takemoto Y, Yamanoi A, Hayashi T, et al. Incidence and factors associated with intrahepatic recurrence following resection of hepatocellular carcinoma. Gastroenterology. 2004; 105: 488- 494.
- Eguchi S, Kanematsu T, Arii S, Okazaki M, Okita K, Omata M, et al. Comparison of the outcomes between an anatomical subsegmentectomy and a non-anatomical minor hepatectomy for single hepatocellular carcinomas based on a Japanese nationwide survey. Surgery. 2011; 143: 469-475.
- Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2009; 242: 252-259.
- Hasegawa K, Kokudo N. Surgical treatment of hepatocellular carcinoma. Surg Today. 2012; 39: 833-843.
- Du ZG, Li B, Wei YG, Yin J, Feng X, Chen X. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011; 10: 265-269.
- Sakka SG, Meier-Hellmann A. Non-invasive liver function monitoring by indocyanine green plasma disappearance rate in critically ill patients. Int J Intensive Care. 2008; 9: 66-72.
- Shindoh J, Hasegawa K, Inoue Y, Ishizawa T, Nagata R, Aoki T, et al. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB (Oxford). 2013; 15: 31-39.
- Bruix J, Hessheimer AJ, Forner A, Boix L, Vilana R, Llovet JM. New aspects of diagnosis and therapy of hepatocellular carcinoma. Oncogene. 2010; 25: 3848-3856.
- Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1988; 7: 462-503.
- Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2006; 89: 500-507.
- Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2007; 38: 200-207.
- Poon RT, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Different risk factors and prognosis for early and late intrahepatic recurrence after resection of hepatocellular carcinoma. Cancer. 2006; 89: 500-507.
- Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, et al. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2005; 101: 796-802.
- Wu CC, Cheng SB, Ho WM, Chen JT, Liu TJ, P’eng FK. Liver resection for hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2005; 92: 348- 355.
- Sakata J, Shirai Y, Wakai T, Kaneko K, Nagahashi M, Hatakeyama K. Preoperative predictors of vascular invasion in hepatocellular carcinoma. Eur J Surg Oncol. 2008; 34: 900-905.
- Kim H, Park MS, Park YN, Kim H, Kim KS, Choi JS, et al. Preoperative radiologic and postoperative pathologic risk factors for early intra-hepatic recurrence in hepatocellular carcinoma patients who underwent curative resection. Yonsei Med J. 2009; 50: 789-795.
- Tarao K, Ohkawa S, Shimizu A, Harada M, Nakamura Y, Ito Y, et al. Significance of hepatocellular proliferation in the development of hepatocellular carcinoma from anti-hepatitis C virus-positive cirrhotic patients. Cancer. 1994; 73: 1149- 1154.
- Ruà S, Comino A, Fruttero A, Torchio P, Bouzari H, Taraglio S, et al. Flow cytometric DNA analysis of cirrhotic liver cells in patients with hepatocellular carcinoma can provide a new prognostic factor. Cancer. 1996; 78: 1195- 1202.
- Morris-Stiff G, Gomez D, de Liguori Carino N, Prasad KR. Surgical management of hepatocellular carcinoma: is the jury still out? Surg Oncol. 2009; 18: 298-321.
- Bigourdan JM, Jaeck D, Meyer N, Meyer C, Oussoultzoglou E, Bachellier P, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl. 2003; 9: 513-520.
- Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997; 25: 87-92.
- Wakai T, Shirai Y, Yokoyama N, Nagakura S, Hatakeyama K. Hepatitis viral status affects the pattern of intrahepatic recurrence after resection for hepatocellular carcinoma. Eur J Surg Oncol. 2003; 29: 266-271.
- Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006; 243: 229-235.
- Du Y, Su T, Ding Y, Cao G. Effects of antiviral therapy on the recurrence of hepatocellular carcinoma after curative resection or liver transplantation. Hepat Mon. 2012; 12: e6031.
- Yang T, Lu JH, Zhai J, Lin C, Yang GS, Zhao RH, et al. High viral load is associated with poor overall and recurrence-free survival of hepatitis B virusrelated hepatocellular carcinoma after curative resection: a prospective cohort study. Eur J Surg Oncol. 2012; 38: 683-691.
- Lok AS. Does antiviral therapy prevent recurrence of hepatitis B virus-related hepatocellular carcinoma after curative liver resection? JAMA. 2012; 308: 1922-1924.
- Shah SA, Greig PD, Gallinger S. Factors associated with recurrence after resection for hepatoceluular carcinoma [J] Am surg. 2006; 202: 275-283.
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999; 229: 216–222.
- Zhao JY, Gu JR, He XH, Wan DF. The study on the function and the molecular mechanism of Ache in hepatic carcinogenesis. Hepatology. 2010; 187: 195-199.
- Yao FD, Dong ZZ, Gu QQ, Yao M. Specific diagnosis of gamma-glutamyl transferase subfraction and its genotyping for hepatocellular carcinoma. Surg Oncol. 2009; 18: 298-321.
- Di-Peng Qu, Lian-Yue Yang, Geng-Wen Huang, Yi-Ming Tao, Xiang Ding, Zhi-Gang Chang. Clinical analysis of the risk factors for recurrence of hepatocellular carcinoma and its relationship with hepatitis virus B infection. World J Gstroenterol. 2005; 11: 2061-2066.
- Zheng-Gui Du, Yong-Gang Wei, Ke-Fei Chen, Bo Li. Risk factors associated with early and late recurrence after curative resection of hepatocellular carcinoma: a single institutions experience with 345 consecutive patients. Hepatobiliary PANCREAT Dis Int. 2014; 13: 153-161.
- Zhao-Xia Duan, Lin-Hua Yang. Research of coagulation, fibrinolysis and matrix metalloproteinase system with cancer metastases and thrombosis. Oncology. 2008; 10: 423-430.