
Research Article
Ann Hematol Oncol. 2019; 6(9): 1267.
PEITC Suppresses the Growth of Lewis Lung Cancer in Immune-Complete Mouse
Liuyang He, Mingming Su, Yu Bai, Xiao Sun, Lei Xia, Chunjian Qi
Medical Research Center, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, China
*Corresponding author: Chunjian Qi, Medical Research Center, The Affiliated Changzhou No. 2 People’s Hospital of Nanjing Medical University, Changzhou, China
Received: July 08, 2019; Accepted: July 30, 2019; Published: August 06, 2019
Abstract
Phenethyl Isothiocyanate (PEITC) isolated from cruciferous vegetables has gained wide attention in recent years due to its strong anticancer effects. Previously, numerous studies have showed that PEITC can induce excessive Reactive Oxygen Species (ROS) accumulation, mitochondrial dysfunction and suppression of stem-like cancer cells, thus leading to the apoptosis of cancer cells and the inhibition of tumor development. However, few studies have analyzed the effects of PEITC on the immune cells of tumor-bearing hosts. In the current study, we found that PEITC treatment slowed down the growth of Lewis lung cancer in immune-complete C57BL/6 mice rather than BALB/cnude mice. Based on the result, we focused on immune cells of Lewis Lung Cancer (LLC)-bearing C57BL/6 mice treated by the oral administration with PEITC and then detecting changes in the number of MDSCs (myeloid-derived suppressor cells), macrophages, T lymphocytes from tumor issues, Draining Lymph Nodes (DLNs) and spleens. Results from flow cytometry analysis and immunocytochemistry showed the number of tumor-infiltrating CD8+T cells of C57BL/6 mice receiving PEITC per day declined significantly when compared with that of untreated control mice, while intra-tumoral MDSCs, macrophages and CD4+T cells were unaffected. These findings suggest that PEITC can exert anticancer function by inhibiting CD8+T cells with immunomodulatory capacity. Our study offers a new theoretical basis for the clinical application of PEITC in lung cancer treatment.
Keywords: PEITC; Lewis lung cancer; Regulatory CD8+T cells; Mice model
Introduction
With an increasing number of smokers and deterioration of the environment, the incidence rate of lung cancer has been increasing worldwide. It is predicted that more than one million Chinese will develop lung cancer yearly by 2025 according to the World Health Organization (WHO)’s estimates. Thus, measures should be taken to prevent and control this health-threatening cancer [1]. In addition to radiotherapy and chemotherapy, how to reactivate the autoimmunity of tumor patients is an effective therapeutic modality against cancer, as the emergence and development of tumors are closely related to the loss of immune function. The ability of immune cells to distinguish between normal cells and cancerous cells is attributed to tumor antigens that are specifically expressed on cancer cells. T effector cells are prime contributors to eradicate tumor cells. Particularly, cytotoxic CD8+T cells can recognize tumor antigens complexed with Major Histocompatibility Class I (MHC-I) on the Antigen- Presenting Cell (APC) surface, thus exerting specific immune-killing effects by generating apoptosis signals (FasL) and releasing perforin and granzymes. However, a relatively small group of CD8-expressing regulatory cells harbors immunosuppressive functions. This cell population has similarities to conventional CD4+Foxp3+Tregs in suppressing immune function and has been studied deeply as noncanonical regulatory cells [2].
JC-5411, a new-targeted antitumor drug produced by Wuxi JC Company Limited, mainly consists of the active component Phenylethyl Isothiocyanate (PEITC) isolated from cruciferous vegetables, such as cauliflower, radish and cabbage. PEITC, which is endowed with the Isothiocyanate (-N=C=S) structure in its molecule, has been widely investigated for its health-protecting benefits, especially regarding its strong cancer-treatment effects on various cancers, including oral cancer [3], head and neck cancer [4], brain glioblastoma [5], and prostate cancer [6]. Therefore, PEITC has been registered in phase III clinical trials in the USA to further confirm the safety and efficacy of tumor therapy. It has been reported that several different mechanisms are involved in preventing and treating cancers with PEITC. A previous study showed that PEITC and its analogs can cause ROS accumulation and redox imbalance in cancer cells to inhibit tumor growth [7]. In another study, only human sensoryacceptable doses of PEITC (10 mg per kg body) were proven to induce cell cycle arrest in the G1/S phase of p53-mutated oral cancer cells [3]. However, limited studies have reported on whether PEITC affects immune cells in mice with tumors. In the only published paper, MDSCs from Peripheral Blood Mononuclear Cells (PBMCs) of breast cancer xenografts in SCID/NSG mice were suppressed with PEITC oral treatment [8].
In our study, we first compared the curative effect of JC-5411 between Lewis Lung Cancer (LLC)-bearing C57BL/6 mice and BALB/c-nude mice. After treating with JC-5411 by oral gavage in established tumor-bearing mice, we found that JC-5411 treatment retarded the growth of tumors in C57BL/6 mice compared with that in BALB/c-nude mice. Next, we focused on the effects of JC-5411 oral administration on multiple leukocytes from the tumor issues, Draining Lymph Nodes (DLNs) and spleens in C57BL/6 mice with Lewis lung cancer. Our results showed that PEITC had an inhibitory effect on tumor-infiltrating suppressive CD8+T cells to delay tumor development.
Materials and Methods
Chemicals, mice and cell lines
JC Pharmaceutical Company Limited (Wuxi, Jiangsu, China) kindly provided JC-5411. Female SPF experimental C57BL/6 mice and BALB/c-nude mice, aged 6-8 weeks, were purchased from Changzhou Cavens Company. All mice were fed with free access to food and drinking water and were maintained under specific pathogen-free conditions. The Lewis Lung Cancer line (LLC) was obtained from the American Type Culture Collection (ATCC) and cultured in Roswell Park Memorial Institute-1640 Medium (RPMI- 1640; Thermo-Fisher Scientific), supplemented with 10% heatinactivated fetal bovine serum (FBS; Thermo-Fisher Scientific). The cells were incubated at 37oC under a humidified atmosphere of 5% CO2. Fluorescein-conjugated mouse-specific antibodies against CD11b, CD4, CD8, Gr-1 and F4/80 for flow cytometry analysis were purchased from BD Pharmingen. The rabbit monoclonal antibody against CD8 for immunocytochemistry and immunofluorescence was purchased from Abcam (ab217344), and goat anti-rabbit IgG cross-adsorbed secondary antibody conjugated with Alexa Fluor 568 was obtained from Thermo-Fisher Scientific.
Animal experiments
Viable Lewis lung cancer cells were counted and suspended in phosphate-buffered saline (PBS; Thermo-Fisher Scientific) at a concentration of 5×106 cells/ml. Next, 100 μl of cells suspended in PBS (including 5×105 cells) were subcutaneously injected into the flanks of each mouse. After the tumor became measurable, the mice were randomly assigned to two groups (control group vs. treatment group). The mice in the treatment group were orally administered with 200 μl of 5 mg/ml of JC-5411 once per day, while the mice in the control group were fed normally without treatment. The tumor masses were measured with a caliper three times per week, and the tumor dimensions were calculated according to the formal V=1/2*A*B2, where A is the vertical length of the tumor and B is the horizontal length of the tumor. At the end of the experiment, all the mice were sacrificed by CO2 overdose in compliance with relevant laws and institutional guidelines. The tumors from two groups of mice were dissected out and weighed. Spleens and Draining Lymph Nodes (DLNs) of mice were dissected out simultaneously for subsequent experiments.
Cell preparation and flow cytometry analysis of leukocytes
Tumor issues from control mice and treated mice were finely minced and digested with collagenase. Briefly, small minced tumor pieces were rinsed twice with PBS solution and were digested on a rotating platform at 37oC for 30 min in 50 ml of 5% FBS/RPMI medium containing 0.25 g of collagenase, 0.1 g of hyaluronidase and 0.0075 g of DNase. After digestion, the tissues were filtered to remove insoluble fiber. The tumor single-cell suspension was washed with 1× Red Blood Cell (RBC) lysis buffer to remove red blood cells. The tumor cells were collected and washed with PBS for surface marker staining. Fluorescein-conjugated Mouse-Specific Antibodies (mAbs) against CD11b, CD4, CD8, Gr-1 and F4/80 were used to label leukocytes. Labeled cells were incubated on ice for 30 min in the dark, followed by washing and analysis by flow cytometry (BD Biosciences) to determine the cell phenotype.
The spleens from mice were ground with a syringe and were washed with PBS. Red blood cells were removed with RBC lysis buffer as described above. The collected single-cell suspensions were stained with mAbs to investigate the effects of JC-5411 on leukocytes.
The Draining Lymph Nodes (DLNs) were ground as spleens without lysis with RBC lysis buffer. The single-cell suspensions were washed with PBS and stained with mAbs for 30 min on ice, followed by analysis using a FACS Canto TM flow cytometer (BD Biosciences). All the samples were analyzed using FlowJo software.
Immunocytochemistry and immunofluorescence
The tumor issues covered with cryo-embedding media were stored at -80oC for sectioning. After the tissue blocks were completely frozen, they were transferred to a cryostat (-20oC) for sectioning and the temperature of the frozen tissue blocks was allowed to equilibrate to that in the cryostat for 15 min. Using a cryotome, the tissue blocks were sectioned into a thickness of 6-8 μm, and sections were placed onto glass slides with positive charges. The glass slides loaded with tissue sections were immersed in precooled methanol at -20oC for 10 min to fix the tissue sections, followed by washing with PBS twice for 5 min. The glass slides were incubated in 0.3% H2O2 dissolved in methyl alcohol at room temperature for 10 min to inactivate endogenous peroxidase and then washed in PBS twice for 5 min each. Subsequently, 100 μl of blocking buffer (5% NGS in TBST) was added onto the sections of the slides, which were then were incubated at room temperature for 1 hour. Excess blocking buffer was drained off from the slides, which were then rinsed with PBS 3 times (5 min each). Next, 100 μl of primary antibodies diluted in blocking buffer (1:400) was added dropwise onto the sections on the slides, which were then incubated in a humidified chamber at room temperature overnight. On the following day, the slides were washed with PBS 3 times to remove excess primary antibodies. Thereafter, 100 μl of diluted secondary antibodies was applied to the sections after equilibration to room temperature. The slides were incubated in a humidified incubator at 37oC for 40 min, followed by washing with PBS for 5 min. Next, 100 μl of DAB substrate solution was applied to reveal the color of the antibody staining. The color developing time was controlled by observation with a microscope. When the desired color intensity of background was reached, the DAB solution was removed quickly, and then the slides were completely washed with running tap water for 10 min.
For immunofluorescence, the tissue blocks were sectioned and fixed for the preparation of immunofluorescence as described above. Prediluted anti-CD8 alpha antibody (1:500 of rabbit monoclonal antibody against CD8) was applied onto the sections of slides. The glass slides loaded with stained tissue sections were incubated at 4oC in the dark overnight. On the following day, the tissue sections were stained with goat anti-rabbit IgG secondary antibody (1:1000) for 40 min, and living cells were stained using diluted DAPI solution (1:500) for 5 min to identify cells. The glass slide was immersed in PBS and washed twice for 5 min each. The CD8+T cells of sections were studied by fluorescence microscopy (U-RFL-T; Olympus).
Statistical analysis
GraphPad Prism 6.0 was used to conduct statistical analysis. The results of animal experiments were represented as the mean values ± SD with a minimum value of n=3. The data were analyzed using Student’s t-test. The differences between groups were considered statistically significant at p‹0.05.
Results
JC-5411 treatment suppresses the growth of tumors in C57BL/6 mice with Lewis Lung Cancer (LLC), but not in BALB/c-nude mice.
To determine whether JC-5411 could exert anti-cancer effects through the immune system in vivo, 5×105 LLC cells cultured in vitro were subcutaneously injected into the inguinal regions of each C57BL/6 female mouse and BALB/c-nude female mouse lacking the thymus, respectively. Once the tumor was palpable, the mice were randomly and equally divided into two groups (control group and JC-5411-treated group). Control mice were fed normally, while mice of the treated group were administered intragastrically one time every day with JC-5411 at a dose of 200 μl (5 mg/ml) additionally. Compared with the control groups, C57BL/6 mice treated with JC- 5411 had smaller tumor volumes and lighter tumor weights at the end of experiment (Figure 1a), while there were no significant differences in the tumor growth and tumor weight between JC-5411-treated BALB/c-nude mice and control BALB-nude mice (Figure 1b). These results indicated that JC-5411 had an inhibitory effect on tumor growth in C57BL/6 mice but no substantial effect on tumor-bearing nude mice, suggesting that JC-5411 could exert immune systemmediated anti-cancer efficiency in vivo.
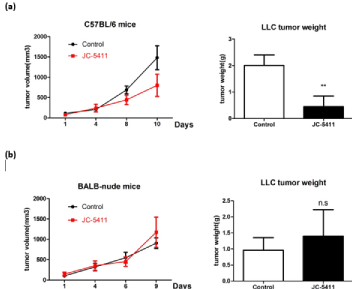
Figure 1: JC-5411 inhibits the growth of Lewis lung cancer in C57BL/6 mice. LLC cells (5×105) were suspended in 100 μL of PBS (per mouse) and were
injected subcutaneously into C57BL/6 female mice and BALB/c-nude female mice. JC-5411 treated mice received 200 μl (5 mg/ml) of JC-5411 by oral gavage
daily; control mice were fed normally without additional administration. Each group contained more than 3 mice. (a) Tumor growth curve and mean tumor weight
of C57BL/6 mice from the two groups at the end of the experiment. (b) Tumor growth curve and mean tumor weight of BALB-nude mice from the two groups at the
end of the experiment. (** means p‹0.01; n.s. means not significant).
JC-5411 treatment affects the T lymphocytes of the spleen but increases the number of T lymphocytes of the Draining Lymph Nodes (DLNs) in LLC mice.
Since JC-5411 treatment did not control the development of Lewis lung cancer in BALB/c-nude mice, we supposed that the immune system participates in tumor suppression caused by JC-5411. Hence, the spleens and DLNs from two mouse groups with LLC were carefully taken out, and single-cell suspensions obtained from spleens and DLNs were prepared for flow cytometry analysis of T lymphocytes. The T cells were stained with specific markers against CD4 and CD8, and the percentages of the two subpopulations of T lymphocytes were determined using FCM. The data showed that JC-5411 treatment had no effects on CD4+T and CD8+T cells in the spleens (Figure 2a) but increased the percentage of CD4+T lymphocytes in DLNs from 11.1% to 20.1%, as well as that of CD8+T lymphocytes from 17% to 24.1% in DLNs (Figure 2b). These observations suggested that JC-5411 may lead to the accumulation of T lymphocytes in DLNs, although T lymphocytes in the spleens were unaffected by JC-5411 treatment.
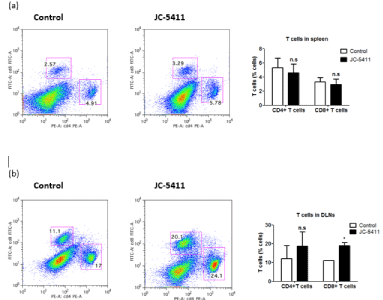
Figure 2: Effects of JC-5411 on T lymphocytes from spleens and DLNs. After JC-5411 oral administration for 10 days, the mice in the control and treated
groups were sacrificed, the spleens and DLNs were carefully dissected out, and single-cell suspensions from issues were stained with specific markers for FACS
analysis. (a) Population of T lymphocytes in the spleens from control mice and JC-5411 treated mice. (b) Population of T lymphocytes in DLNs from control mice
and JC-5411 treated mice. (* means p‹0.05; n.s. means not significant).
JC-5411 treatment reduces the number of tumor-infiltrating CD8+T cells but has no effects on tumor-infiltrating CD4+T cells, macrophages and Myeloid-Derived Tumor Suppressor Cells (MDSCs).
PEITC was reported to inhibit the growth of xenograft tumors in mice by reducing MDCSs from Peripheral Blood Mononuclear Cells (PBMCs) [8]. However, whether PEITC could exert an influence on tumor-infiltrating immune cells to exploit the anti-cancer function remains unclear. We prepared single-cell suspensions from tumor tissues and used mouse-specific antibodies to detect multiple immune cells. The relative percentages of various populations of macrophages, MDSCs and T lymphocytes were determined using flow cytometry. As shown in Figure 3a, no obvious differences were observed in the proportion of intratumoral CD11b+F4/80+ macrophages between control mice and JC-5411-treated mice. Similarly, JC-5411 treatment also did not significantly change the quantity of CD11b+Gr1+ MDSCs in cancer tissues. In terms of intratumoral lymphocytes, the proportion of tumor-infiltrating CD8+T cells decreased significantly from 10.7% to 1.35% with JC-5411 treatment, whereas the percentage of tumor-infiltrating CD4+T cells remained unchanged generally compared with that of control mice (3.21% vs. 3.41%) (Figure 3a).
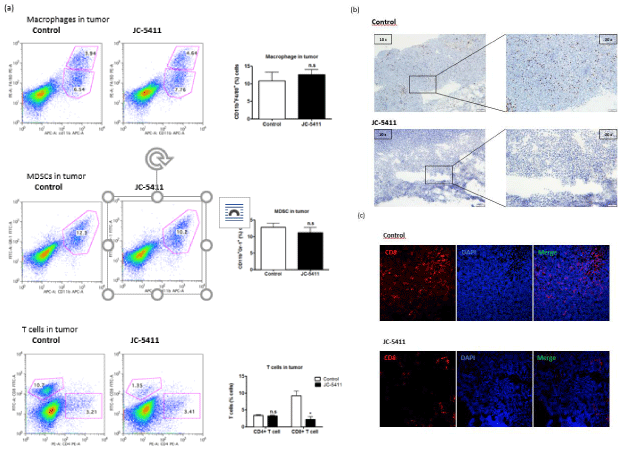
Figure 3: Effects of JC-5411 on the multiple populations of tumor-infiltrating leukocytes. Tumors in JC-5411-treated versus control mice with LLC were
analyzed by flow cytometry, immunocytochemistry and immunofluorescence. Single-cell suspensions from tumor tissues were stained with specific markers
to detect leukocytes using FACS analysis. Lymphocyte population in tumor samples collected from two groups of mice was gated for subsequent analysis of
subpopulation with Fluorescein-conjugated mouse-specific antibodies. (a) Population of tumor-infiltrating macrophages, MDSCs and T cells in control mice and
JC-5411-treated mice. (* means p‹0.05; n.s. means not significant) (b) Representative microscopic figures of LLC tumor tissues immunocytochemically stained for
CD8. Two images of the left panel were obtained at 10× magnification, and two images of the right panel were obtained at 20× magnification. (c) Representative
microscopic figures of LLC tumor tissues detected by immunofluorescence staining for CD8 and DAPI. All images were obtained at 20× magnification.
To confirm the results from flow cytometry analysis, immunohistochemical staining (Figure 3b) and immunofluorescent staining (Figure 3c) were performed to further identify the CD8+T cells of tumor tissues between control mice and experimental mice. Consistent with the finding of FACS analysis, we found a substantial decline in the number of CD8+T cells of LLC tumors from mice with JC-5411 oral administration. Taken together, JC-5411 had a suppressive effect on CD8+T cell infiltration in tumor tissues, although the number of macrophages, MDSCs and CD4+T cells was unaffected by JC-5411 treatment. These observations indicated that JC-5411 may be involved in the CD8+T cells-mediated anti-tumor mechanism.
Discussion
Sulforaphane has been extensively studied for its robust anticancer effects on multiple tumor types due to the structure of the Isothiocyanates (ITCs) [9]. The most important anticancer effect mainly depends on its ability to generate intracellular excessive Reactive Oxygen Species (ROS), which was reported to cause oxidative stress and induce the apoptosis of malignant tumor cells [10]. The structural variations of the ITC side chain may also influence the anticancer activity. The presence of an aromatic ring on the ITC side chain (e.g., PEITC) causes microtubule depolymerization in HepG2 cells without changing the morphology of DNA [11]. Another PEITC analog with an ethylamide group at the para position of the benzene ring of PEITC (LBL21) was proven to possess better anti-proliferative effects than PEITC and to eliminate Side Population (SP) cells that are considered Cancer Stem Cells (CSCs) because of their stem-like properties [12]. JC-5411 (trade name Abrassin), a new antitumor medicine developed by Wuxi JC Company Limited to target CSCs and reduce drug resistance, has an identical chemical structure as PEITC and was prepared into capsules for sales. On the one hand, JC-5411 was reported to decrease the Glutathione (GSH) level and increase the ROS level of targeted CSCs, thus triggering cellular redox imbalance and inducing CSC death. On the other hand, JC-5411 serves to inhibit drug efflux by lowering the permeability of the CSC membrane, resulting in the apoptosis of CSCs.
It is well accepted that the occurrence of tumors is thought to be linked to the loss of immune function of tumor-bearing hosts. Generally, two main forces are associated with the adaptive immune system to affect tumorigenicity–cytotoxic CD8+T cells and regulatory T cells (Tregs) [13]. The vital contributor to the elimination of tumor cells is cytotoxic CD8+T cells, which can form the complex of tumor antigen peptide and MHC-I expressed on the surfaces of APCs by the T-Cell Receptor (TCR). Once CD8+T cells recognize and target tumor cells, these effector cells can induce the lysis of targeted cells by releasing cytotoxins such as perforin, granzymes and cytokines. There is substantial evidence showing that the high density of tumorinfiltrating CD8+T cells is a favorable indicator of clinical prognosis in tumor patients [14,15]. Unlike cytotoxic CD8+T cells that can kill cancer cells, Tregs are pro-tumoral and play an opposite role in limiting the growth of tumors. Conventional CD4+Foxp3+regulatory T cells with a suppressive immune function were extensively studied by researchers all worldwide [16]. Some studies have also revealed that the low ratios of CD8+T cells to CD4+CD25+Foxp3+Tregs indicate a positive correlation with a poor clinical outcome and overall survival in ovarian [17] and breast cancers [18]. Bone marrow-derived MDCSs with tumor-promoting effects also attracted researchers’ increasing attention because this cell population can stimulate the expansion of regulatory CD4+T cells [19,20]. Although an important mechanism proposed to explain the anticancer effects was PEITC-mediated suppression of PBMC-derived MDSCs that can cause an increase in regulatory CD4+T lymphocytes in mice with MDA-MB-231 breast cancer xenografts [8], we observed no alteration in MDSCs and CD4+T cell infiltration in the cancer mass after treating LLC mice with JC-5411 oral medication.
Surprisingly, our results showed that JC-5411 oral treatment reduced the number of tumor-infiltrating CD8+T cells that were previously shown to be the key effector cell population against tumors, rather than immunosuppressive cells such as MDSCs or CD4+Tregs. However, CD8-expressing Tregs are in the spotlight and proved to be a potent regulatory sublineage in tumor immunology. A study showed recently that the differentiation and activation of CD8+Tregs may be triggered by the glycolysis pathway [21]. Various mechanisms concerning the immunosuppressive functions of CD8+Tregs were explored. Klimiuk et al., found that the suppressive activity is associated with the production of IL-16 in severe combined immunodeficiency mice [22]. In another study conducted by Notley et al., the proportion of CD8+CD25+Foxp3+ Tregs was increased by using an anti-CD3 antibody in mice with collagen-induced arthritis [23]. Although studies on CD8+Tregs are in progress, a deep understanding of CD8+Tregs remains limited due to the expression of multiple effector surface markers. The most common CD8+Tregs can be divided into two subpopulations depending on the expression of the costimulatory receptor CD28: CD28lowCD8+Tregs and CD28-CD8+Tregs [24]. A report showed that trichosanthin-derived peptides could attenuate autoimmune Encephalomyelitis (EAE) in mice due to the activation of IL 10-producing CD8+CD28low Tregs [25]. A human study also demonstrated that Peripheral Blood Mononuclear Cell (PBMC)-derived CD8+CD28 low T cells that can produce IL-10 and TGF-β are immunosuppressive [26]. CD8+CD28-Tregs with immunomodulatory activity have been observed in patients with various cancers such as nonsmall cell lung cancer [27] and ovarian cancer [28]. Based on our research results and theories, we consider that tumor-infiltrating CD8+T cells are inhibited by JC-5411 as regulatory CD8+T cells but not cytotoxic CD8+T lymphocytes, contributing to the elimination of malignant tumor cells. Hence, we conclude that the antitumor function of JC- 5411 may be mediated through the inhibition of regulatory CD8+T cells in Lewis lung cancer.
In summary, we showed that JC-5411 oral treatment may slow down LLC tumor development in C57BL/6 mice but not nude mice, reporting a novel antitumor mechanism of PEITC using follow-up molecular investigation. From the animal experiments, we speculate that the anticancer property of JC-5411 is associated with the function of the immune system and confirm that the anticancer activity of JC- 5411 depends on the reduction of intratumoral regulatory CD8+T cells. This finding merits further studies to identify this population of CD8+T cells using a surface-specific marker and evaluate the value of JC-5411 in cancer treatment.
Funding
National Natural Science Foundation of China (81672799 to C.Q.) and the Changzhou High-Level Health Personnel Training Project (2016CZLJ016 to C.Q.) supported this work.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: a cancer journal for clinicians. 2005; 55: 74-108.
- Zhao H, Feng R, Peng A, Li G, Zhou L. The expanding family of noncanonical regulatory cell subsets. Journal of leukocyte biology. 2019.
- Lam-Ubol A, Fitzgerald AL, Ritdej A, Phonyiam T, Zhang H, Myers JN, et al. Trachootham D: Sensory acceptable equivalent doses of beta-Phenylethyl Isothiocyanate (PEITC) induce cell cycle arrest and retard the growth of p53 mutated oral cancer in vitro and in vivo. Food & function. 2018; 9: 3640-3656.
- Eastham LL, Howard CM, Balachandran P, Pasco DS, Claudio PP. Eating Green: Shining Light on the Use of Dietary Phytochemicals as a Modern Approach in the Prevention and Treatment of Head and Neck Cancers. Current topics in medicinal chemistry. 2018; 18: 182-191.
- Chou YC, Chang MY, Wang MJ, Yu FS, Liu HC, Harnod T, et al. PEITC inhibits human brain glioblastoma GBM 8401 cell migration and invasion through the inhibition of uPA, Rho A, and Ras with inhibition of MMP-2, -7 and -9 gene expression. Oncology reports. 2015; 34: 2489-2496.
- Khor TO, Keum YS, Lin W, Kim JH, Hu R, Shen G, et al. Combined inhibitory effects of curcumin and phenethyl isothiocyanate on the growth of human PC-3 prostate xenografts in immunodeficient mice. Cancer research. 2006; 66: 613-621.
- Wang J, Luo B, Li X, Lu W, Yang J, Hu Y, et al. Inhibition of cancer growth in vitro and in vivo by a novel ROS-modulating agent with ability to eliminate stem-like cancer cells. Cell death & disease. 2017; 8: e2887.
- Gupta P, Wright SE, Srivastava SK. PEITC treatment suppresses myeloid derived tumor suppressor cells to inhibit breast tumor growth. Oncoimmunology. 2015; 4: e981449.
- Bayat Mokhtari R, Baluch N, Homayouni TS, Morgatskaya E, Kumar S, Kazemi P, et al. The role of Sulforaphane in cancer chemoprevention and health benefits: a mini-review. Journal of cell communication and signaling. 2018; 12: 91-101.
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROSmediated mechanisms: a radical therapeutic approach? Nature reviews Drug discovery. 2009; 8: 579-591.
- Pocasap P, Weerapreeyakul N, Thumanu K. Structures of isothiocyanates attributed to reactive oxygen species generation and microtubule depolymerization in HepG2 cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2018; 101: 698-709.
- Liu PP, Liao J, Tang ZJ, Wu WJ, Yang J, Zeng ZL, et al. Metabolic regulation of cancer cell side population by glucose through activation of the Akt pathway. Cell death and differentiation. 2014; 21: 124-135.
- Gun SY, Lee SWL, Sieow JL, Wong SC. Targeting immune cells for cancer therapy. Redox biology. 2019: 101174.
- Vano YA, Petitprez F, Giraldo NA, Fridman WH, Sautes-Fridman C. Immunebased identification of cancer patients at high risk of progression. Current opinion in immunology. 2018; 51: 97-102.
- Church SE, Galon J. Regulation of CTL Infiltration within the Tumor Microenvironment. Advances in experimental medicine and biology. 2017; 1036: 33-49.
- Ramsdell F, Ziegler SF. FOXP3 and scurfy: how it all began. Nature reviews Immunology. 2014; 14: 343-349.
- Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PloS one. 2013; 8: e80063.
- Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8(+) cytotoxic T cell and FOXP3(+) regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast cancer research and treatment. 2011; 130: 645-655.
- Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological reviews. 2008; 222: 162-179.
- Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE, 3rd. Myeloid-derived suppressor cells in breast cancer. Breast cancer research and treatment. 2013; 140: 13-21.
- Zhang S, Wu M, Wang F. Immune regulation by CD8 (+) Treg cells: novel possibilities for anticancer immunotherapy. Cellular & molecular immunology. 2018; 15: 805-807.
- Klimiuk PA, Goronzy JJ, Weyand CM. IL-16 as an anti-inflammatory cytokine in rheumatoid synovitis. Journal of immunology. 1999; 162: 4293-4299.
- Notley CA, McCann FE, Inglis JJ, Williams RO. ANTI-CD3 therapy expands the numbers of CD4+ and CD8+ Treg cells and induces sustained amelioration of collagen-induced arthritis. Arthritis and rheumatism. 2010; 62: 171-178.
- Vuddamalay Y, Van Meerwijk JP. CD28 (-) and CD28 (low) CD8(+) Regulatory T Cells: Of Mice and Men. Frontiers in immunology. 2017; 8: 31.
- Yang N, Li Z, Jiao Z, Gu P, Zhou Y, Lu L, et al. A Trichosanthin-derived peptide suppresses type 1 immune responses by TLR2-dependent activation of CD8 (+) CD28 (-) Tregs. Clinical immunology. 2014; 153: 277-287.
- Vuddamalay Y, Attia M, Vicente R, Pomie C, Enault G, Leobon B, et al. Mouse and human CD8 (+) CD28 (low) regulatory T lymphocytes differentiate in the thymus. Immunology. 2016; 148: 187-196.
- Chen C, Chen D, Zhang Y, Chen Z, Zhu W, Zhang B, et al. Changes of CD4+CD25+FOXP3+ and CD8+CD28- regulatory T cells in non-small cell lung cancer patients undergoing surgery. International immunopharmacology. 2014; 18: 255-261.
- Zhang S, Ke X, Zeng S, Wu M, Lou J, Wu L, et al. Analysis of CD8+ Treg cells in patients with ovarian cancer: a possible mechanism for immune impairment. Cellular & molecular immunology. 2015; 12: 580-591.