
Research Article
Ann Hematol Oncol. 2019; 6(10): 1275.
Obesity and Metabolic Syndrome among Adult Lymphoma Survivors
Daniele A*, De Summa S, Lerario G, Divella R, Ditonno P, Ciavarella S, Paradiso AV, Sciacovelli AM, De Luca R, Savino E, and Guarini A*, Minoia C*
National Cancer Institute, IRCCS Istituto Tumori “Giovanni Paolo II” Bari, Italy
*These Authors contributed equally as last authors in this study
*Corresponding author: Antonella Daniele, National Cancer Institute, IRCCS Istituto Tumori “Giovanni Paolo II” Bari, Italy Viale Orazio Flacco 65, 70124 Bari, Italy
Received: October 13, 2019; Accepted: November 26, 2019; Published: December 03, 2019
Abstract
Background: Metabolic dysfunction, including the metabolic syndrome (MetSyn) and insuline resistance, is a long-term complication of curative treatment for many cancer patients including lymphoma survivors. In these patients, prognosis and quality of life can be adversely influenced by obesity, physical inactivity and metabolic dysfunction so, preventive measures, including dietary counseling and tailored exercise should be initiated early in the course of survivorship.
Patients and Methods: The study was conducted analyzing, in relation to the diagnosis of Diffuse Large B-cell Lymphoma (DLBCL), Hodgkin Lymphoma (HL) and therapy, the presence of MetSyn, obesity, sarcopenia, and type IIA and IIB dyslipidemia. We prospectivelly enrolled 111 lymphoma survivors aged between 24 and 82 years in continuous remission of lymphoma for at least 3 years within the “CCM2014” project supported by the Italian Ministry of Health. Each of them underwent an anthropometric and plicometric evaluation and measurement of metabolic and inflammation parameters (glycaemia, total cholesterol and HDL, triglycerides, C-reactive protein and β-2 microglobulin).
Results: Our results showed a lower risk of developing MetSyn in HL patients than in DLBCL (p‹0.001), while steroid use during therapy significantly increased the risk of MetSyn and sarcopenia in DLBCL patients. Concerning the dyslipidemia IIa, multivariate analysis showed that the HL group had asignificantly lower risk than DLBCL of developing these conditions. Dyslipidemia IIb showed instead of being related to smoking. Particularly, in the univariate analysis both ex-smokers and smokers have a significantly higher risk of developing this metabolic disorder. Anova test, showed in the DLBCL group a statistically significant correlation concerning the waist circumference in both women and men with MetSyn no MetSyn with p›0.05 and p=0.005 respectively; statistically significant association was also observed in the percentage of lean mass; DLBCL men in the group with MetSyn had a significant sarcopenia compared to those without MetSyn (p = 0.04).
Conclusion: These data suggest that DLBCL patients have a higher risk of developing MetSyn and sarcopenia compared to HL, most likely as a result of taking steroids, so an early nutritional intervention associated with adequate physical activity could reduce the risk of onset of both complications in lymphoma survivors. These conclusions had never been previously reported in an Italian or European population of lymphoma survivors, remarking that, also in Countries in which the Mediterranean diet is diffused, these kinds of complications could occur.
Keywords: Metabolic syndrome; Obesity; Lymphoma survivors
Introduction
Malignant lymphomas represent the fifth type of cancer by frequency in the Western World, with an incidence of about 19-20 cases per 100,000 inhabitants per year [1]. The incidence of lymphomas is largely influenced by geographical, racial and temporal factors and is higher in industrialized countries, in male and white race. Survival rates for lymphoma have gradually increase; age-adjusted 5-year survival for HL in adults is about 81% and for Non-Hodgkin’s Lymphomas (NHL) about 60% [2,3]. With increasing numbers of cancer survivors, attention has been drawn to long-term complications of curative cancer treatment, including a range of metabolic disorders. Treatment relatedobesity and metabolic syndrome in adult survivors to lymphoma are risk factor for cardiovascular disease. Both conditions often begin during therapy. The Term Metabolic Syndrome (MetSyn), previously known as syndrome X, defines a cluster of physiological, biochemical and metabolic factors that directly increase the risk of developing atherosclerotic cardiovascular disease, diabetes mellitus and all causes of mortality [4]. Among the various diagnostic criteria proposed, the most widely accepted are those established by the National Cholesterol Education Program Adult Treatment Panel III [5]. The prevalence of the MetSyn increases with age and varies based on genetic factors. Increasing evidence shows that the MetSyn and its components such as obesity and insulin resistance are associated with cancer and treatment [6-8]. Obesity is one of the biggest public health problems on the level worldwide, as it concerns both the populations of the industrialized countries and those of the developing countries. It is estimated that at least 250 million people worldwide are currently suffering from obesity. It is a chronic disease with multifactorial pathogenesis, characterized by an excessive accumulation of body fat, with a consequent increase in weight, resulting from the interaction between predisposing genetic factors and favorable environmental factors [9]; the latter are mainly represented by a sedentary lifestyle, a diet rich in fats and simple sugars, on the whole, by an imbalance between income and energy expenditure. Excessive amounts of free fatty acid lead to insulin resistance and glucose intolerance, and the adipose tissue is the main source of variety of proinflammatory cytokines that may contribute to the development of cardiovascular changes. The increased adipose tissue, which today is considered a true endocrine/autocrine organ, is responsible for an abnormal secretion of free fatty acids and peptides involved in various regulatory functions, which contribute to the development of typical complications of overweight, such as cardiovascular diseases, type 2 diabetes mellitus, hyperlipidemia and hepatic steatosis and Nonalcoholic Fatty Liver Disease (NAFLD) [10].
Insulin resistance is the condition in which insulin-mediated glucose uptake is impaired and this is considered to be the primary event in the development of these metabolic disturbances; it is present in patients with non-insulin dependent diabetes mellitus or impaired glucose tolerance, but can also be found in individuals with normal glucose tolerance. The recent proposed criteria for MetSyn suggest the role of obesity and visceral adipose tissues are associated to insulin resistance [11].
Although the same aetiologic factors as for the general population can be assumed to play a role in the development of the metabolic syndrome in cancer survivors, other explanations can be suggested in the development of this syndrome in these patients. Disturbances of the hypothalamic-pituitary axis and their target organs, like deficiencies of growth hormone and gonadal hormones, have been linked to the development of the metabolic syndrome [12-16]. Some forms of cancer treatment can have harmful effects on the functioning of the hypothalamic-pituitaryaxis and the endocrine organs [17,18]. Therefore, it is conceivable that the occurrence of endocrine disorders after curative cancer treatment is important for the development of the metabolic syndrome in these long-term cancer survivors. Local treatments (surgery and radiotherapy) and systemic cancer therapy (chemo and hormone therapy) can cause changes in endocrine and metabolic functions that might contribute to the development of the metabolic syndrome.
Several authors have demonstrated a role of Growth Hormone (GH) in metabolic syndrome onset as GH stimulates proteinsynthesis and contributes to lipolysis and it indirectly exerts insulinlike effects, such as stimulation of glucose uptake into peripheral tissue, via stimulation of IGF-1 production by the liver; decreased levels of this factor is associated with change in body composition, such as reduction in lean body mass and increase in fat mass which are associated with dyslipidaemia and insulin resistance [19].
A relevant question is whether the MetSyn is more prevalent in cancer survivors after cancer treatment respect to healthy population and, in fact, different authors have evaluated this aspect.
In 1996, Talvensaari et al. described a role of GH with combination of obesity, hyperinsulinaemia and reduced high-density lipoprotein (HDL) cholesterol, in a group of long term survivors of childhood cancer who were mainly given a combination of chemotherapy and radiotherapy reportingan increased risk of the metabolic syndrome in survivors respect to healthy control. Growth-hormone deficiency is the common endocrine dysfunction in survivors treated with radiotherapy and is associated with obesity [20].
MetSyn seems to be an emergent complication of NHL survivors, as shown in a meta-analysis performed in 2012, and of patients who had undergone a hematopoietic stem cell transplant (autologous or allogeneic) [21,22]. In the subset of lymphoma survivors, Body Mass Index (BMI) has been more frequently analyzed, due to its easier evaluation. In different series, both HL and NHL survivors present an increased BMI in comparison to the general population [23,24], but this parameter cannot be considered as a surrogate for MeSyn.
The aim of this study was to assess in a prospective study the prevalence MeSyn in lymphoma survivors in order to offer an early and adequate nutritional support to reduce the risk of secondary neoplasms, metabolic disorders and cardiovascular late events.
Patients and Methods
This is a monocentric study conducted from November 2016 to July 2018; we enrolled 111 consecutive patients (62 women and 49 men), median age 51 years (range, 22-82) in continuous remission of both NHL and HL, for at least 4 years and in current follow-up at our Institution as part of the “Centre for Disease Control Program 2014 CCM2014) launched by the Italian Ministry of Health. The study was approved by the local Ethical Commettee and patients willing to participate signed informed consent. Clinical data related to the primary onco-haematological disease and anti-neoplastic treatment were obtained by electronic database. In the context of an onco-haematological follow-up visit, each patient was subjected to nutritional evaluation of the bi-compartment by anthropometry and plicometry and a questionnaire was administered to each of them to assess lifestyles (physical activity, smoking, use of alcohol, adherence to specific food models such as Mediterranean, Vegan Vegetarian, etc.) [25], pharmacological therapies in progress, pathological states of nutritional interest, food allergies or intolerances, daily food intake and/or eating behavior disorders, possible dependence on opioids, tranquilizers or sedatives and sleep disorders.
Anthropometry and Plicometry measurement
Weith and heith of all patients were measured with diagnostic device (Millennium 3 DAVI & CIA – Barcelona, Spain).
At the same time, the right and left circumferences (cm) of the wrist, forearm and arm, shoulders, thorax, waist, abdomen, hips, median of the thigh and median of the calf were measured (professional meter BMI, GIMA, Gessate (MI)- Italy) according the standard techniques described by Lohman [26]. The World Health Organization (WHO) definitions of obesity (body mass index, BMI›30 kg/m2 and overweight (BMI 25-29.9kg/m2) were used [27]. The percentage of Fat Mass (FT) and Lean Mass (LM) was carried out using a FAT-1 plicometer (GIMA- Italy) which measures the thickness of skin folds in various districts and assesses the nutritional status of the subject under examination and the sectoral distribution of its adipose tissue. The Durnin-Womerslay measurement of 7 folds (bicipital, tricipital, axillary, subscapular, abdominal, over-iliac and median thigh) was performed [28]. The Basal Energy Expenditure (BEE) has been calculated according to the Harris & Benedict Equation (HBE): for male gender: 66.47 + (13.75 x weight in kg) + (5.003 x height in cm) - (6.775 x age in years). For female gender, the following equation was utilized: 655.09 + (9.563 x weight in kg) + (1.85 x height in cm) - (4.676 x age in years) [29]. The ideal weight of each patient was calculated using the Lorenz formula which includes the following calculation: for male gender: (height in cm -100) – (height in cm -150) /4. For female gender: (height in cm -100) – (height in cm -150) /2.
Blood samples assay
Following overnight fasting, blood samples for measurement of triglyceridae, glycaemia, HDL- cholesterol, total cholesterol, β-2 microglobulin, albumin, C-reactive protein were collected after overnight fasting using COBAS c311/501 (Roche-Diagnostic Limited S.p.A Basel- Switzerland); Definition of the MetSyn was done according to the National Cholesterol Education program Adult treatment Panel III guidelines defined as at least three of the following parameter: [4] waist circumference›102 cm in men and ›88 in women, blood pressure ›130/85 mm/Hg, hypertrigly ceridaemia, hyperglycaemia and lower HDL-cholesterol. The classification of dyslipidemia was performed according to Fredrickson’s phenotypes accepting from World Health Organization (WHO).
Statistical analysis
Data were analyzed by using statistical analysis and size power analysis and graphic (NCSS-PASS) 2007 software. The serum levels of anthropometric and metabolic parameters are expressed as mean±standard deviation.
The correlation between the serum levels of metabolic parameter in two groups (NHL and HL) of patients was performed by t-test while the correlations with anthropometric parameters were performed by Analysis Of Variance (ANOVA). A p value of less than 0.05 was considered as statistically significant.
Univariate and mutivariate logistic regression were performed through generalized linear model, using binomial family and logit link. The “MASS” R package was used in the studio Version 1.1.447 [25]. Results are reported as hazard ratio and 95% confidence interval and they were considered significant when p-value ‹ 0.05.
Results
In this study we enrolled 57 NHL of whom 42 Diffuse Large B-cell lymphoma, 1 Burkitt lymphoma, 1 Primary Central nervous system lymphoma, 1 Mantle cell lymphoma, 7 Follicular lymphoma, 2 Small lymphocytic lymphoma, 2 Marginal zone lymphoma, 1 Anaplastic large cell lymphoma CD30+ ALK+ and 55 classical HL (31 women and 24 men) in current follow-up at our Institute, with a median of 8 years since remission of the disease (range: 4-48). NHL patients had been treated in the majority of cases with CHOP (cyclophosphamide, vincristinem doxorubicin, prednisone) or CHOP-like regimes which included high-dose steroids. HL patients had been treated with ABVD (doxorubicin, bleomicin, vinblastine, dacarbazine) with or without radiotherapy. The 14.9% (n. 17) received a second line chemotherapy, which for 5.2% (n.6) was followed by a autologous stem cell transplant. The baseline clinical-anthropometric and lifestyle habits of 111 lymphoma patients are shown in (Table 1). The correlation between the mean of the anthropometric-plicometric values and the metabolic parameters expressed in mean ± SD was carried out used the analysis of variance (ANOVA) considering significant p-value ‹of 0.05.
Gender
n°
%
Male
49
43.0
Female
65
57.0
Years
(range)
Male
(25-82)
Female
(24-76)
Diagnosis
n°
%
HL
54
49.0
DLBCL
57
51.0
Treatment
n°
%
Chemotherapy
117
100
Autologous transplantation
6
5.2
High dose steroids
n°
%
Yes
66
58.0
No
48
42.0
Hypertension
n°
%
Yes (BP >130/85 mmHg)
51
45.0
No
63
55.0
Weight Status
n°
%
Obesity
40
35.0
Overweight
34
29.8
Normal Weight
38
33.0
Underweight
2
3.0
Byotipe
n°
%
Ginoide
57
50.0
Android
29
26.0
Intermediate
28
24.0
Metabolic syndrome
n°
%
Yes
52
46
No
58
54
Excess malnutrition
n°
%
Yes
65
57.0
No
35
43.0
Eating behavior disorder
n°
%
Yes
54
47.0
No
60
53.0
Metabolic risk
n°
%
High
58
51.0
Slight
33
29.0
Low
23
20.0
Sarcopenia
n°
%
Yes
32
28
No
82
72
Smoke
n°
%
Smoker
23
21.0
Non smoker
58
50.0
Ex smoker
33
29.0
Physical Activity
n°
%
Yes
53
47.0
No
61
53.0
Table 1: Baseline clinical- anthropometric and lifestyle characteristic of 111 Lymphoma survivors.
In the group of NHL survivors 34/59 (57%) were suffering from MetSyn; among these patients a significant correlation was observed between the mean waist circumference (cm), higher in women with MetSyn (94 ± 17) compared to women without MetSyn (84 ± 11) p ‹0.05 as well as in men with MetSyn (104 ± 9.0) compared to those without MetSyn (93 ± 8.1) p = 0.005. Statistically significant association was also observed in the percentage of lean mass, the men of the NHL group with MetSyn had a reduction in lean mass compared to those without MetSyn with averages of 67.9% (± 4.1) and 71.7% (± 5.0) respectively (p = 0.04). HL survivors presented a reduced incidence of MetSyn, which was observed in the 32% of cases.
Regarding serum metabolic parameters, ANOVA test showed a significant correlation for glycaemia, total cholesterol, significantly higher triglycerides in HL patients with MetSyn vs compared to those without MetSyn (p‹0.001).
Finally, a significant correlation was observed in NHL for β-2microglobulin with higher values in patients with MetSyn compared to those without (1.08 ± 0.45 vs 1.82 ± 2.0: p ‹0.001). Date showed in table II.
NHL (N=57)
HL (N=54)
MetS/yes (N= 34)
MetS/no
p value
MetS/yes
MetS/no
p value
(N=23)
(N= 18)
(N=36)
Antropometry
Weight (Kg)
76.3±14.0
73.0±13.0
n.s
75.4±12.5
76.0±19.0
n.s
Hight (cm)
169.5±2.5
166.5±6.7
n.s
166.2±6.1
165.6±17.0
n.s
BMI (kg/h2)
27.5±4.5
26.6±4.2
n.s
26.7±4.0
27.2±6.4
n.s
Waist circumference
Women
94.0±17.0
84.0±11.0
0.05
87.04±12.2
80.9±17.0
n.s
Men
Percentage body fat
104.0±9.0
93.3±8.1
0.005
99.8±12.0
93.5±21.0
n.s
Women
39.5±5.0
38.4±2.6
ns
37.3±4.9
35.0±11.3
n.s
Men
32.1±4.2
28.2±5.0
0.04
29.7±5.1
27.13±10.6
n.s
Percentage Fat Free Max
Women
60.6±4.0
61.5±2.5
0.45
61.8±3.2
63.1±13.1
n.s
Men
67.9±4.1
71.7±5.0
0.04
68.8±5.6
70.3±14.2
n.s
TDEE
2113±309
2185±275
1963±306
2169±345
n.s
Inflammatory markers
CPR (mg/dl)
2.10±2.02
1.90±1.01
n.s
2.25±0.80
1.95±0.99
n.s
B2-microglobulin (mg/L)
1,08±0.45
1.82±0.45
0.001
1.92±0.77
1.82±0.67
n.s
Metabolic parameters
Glycemia (mg/dl)
101.5±17.0
99.3±7.2
n.s
106.6±16.8
101.8±17.7
<0.001
Total cholesterol (mg/dl)
201.0±30.8
199.0±21.0
n.s
209.9±19.8
179.9±10.9
< 0.001
HDL-cholesterol (mg/dl)
52.4±11.9
53.0±11.0
n.s
50.3±27.0
52.2±25.0
n.s
Triglycerides(mg/dl)
133.2±12
130.3±6.0
n.s
135.2±5.8
125.2±10
<0.001
Albumin (%)
55.5±5.3
56.1±4.7
n.s
53.2±3.8
60.8±5.5
0.001
TRF
250,5±35.0
270.7±31.0
n.s
295±28.0
314.0±27
0.001
HCT
41.7±3.8
43.5±2.5
n.s
42.8±1.8
40.8±2.8
0.009
Table 2: Mean value comparison between anthropometric and metabolic parameters in NHL and HL lymphoma patients with or without metabolic syndrome.
Besides we analyzed the presence of MetSyn, dyslipidemia IIa and IIb, the use of steroids during therapy, lifestyle parameters (smoke and physical activity), nutritional comorbidities (e.g, thyroid defects) sarcopenia and food allergiesin relation to diagnosis (HL/NHL).
The univariate logistic regression confirmed a lower risk of MetSyn in HL patients (HR: 0.37, p-value: 0.01) respect to NHL patients. The use of steroids, as part of the induction or second line chemotherapy, significantly increased the risk to be affected by MetSyn (in DLBCL particularly) as we observed also for smoke nutritional comorbidities and sarcopenia. However, food allergy decreases such a risk probably due to limitation on diet (Table 3). Multivariate logistic regression retained as independent factor only sarcopenia respect to MetSyn (Figure 1). Precisely, other factors (diagnosis, use of steroids, smoke, physical activities, and food allergy) being equal, while to be affected by sarcopenia significantly increase the risk to have MetSyn (HR: 3.51; p-value: 0.01).
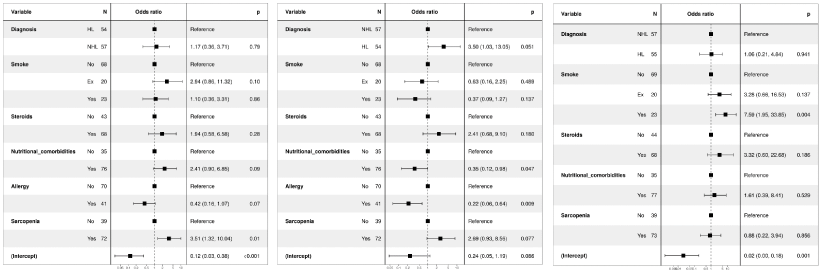
Figure 1: Forest Plot Representing Multivariate Logistic regression for a) Metabolic Syndrome b) Dislypidemia A c) Dislypidemia B.
Odds ratio (95% CI)
p-value
Metabolic syndrome
Diagnosis
NHL
Ref
HL
0.37 (0.16÷0.78)
0.01
Smoke
No
Ref
Ex-smoker
1.29 (0.49÷3.38)
0.58
Yes
5.05 (1.74÷17.11)
0.004
Steroids
2.82 (1.29÷6.51)
0.01
Sarcopenia
5.37 (2.26÷14.02)
0.002
Nutritional comorbidities
2.94 (1.28÷7.22)
0.01
Food allergy
0.36 (0.15÷0.8)
0.01
DyslipidemiaIIa
Foodallergy
0.31 (0.1÷0.8)
0.02
Dyslipidemia IIB
Smoke
No
Ref
Ex-smoker
7.17 (1.94÷30.49)
0.003
Yes
4.09 (0.89÷19.23)
0.06
Table 3: Univariate Logistic regression for MetS, Dyslipdemia IIa and Dyslipdemia IIb.
As observed for MetSyn, the risk to be affected by dyslipidemia IIa is lower in presence of food allergy both in univariate and multivariate analyses. Regarding the type of diagnosis, in multivariate logistic regression it was observed a statistical significance showing that HL patients had a lower risk than DLBCL to have dyslipidemia IIa. Interestingly, a statistical trend (p-value: 0.07) related to an increased risk associated to dyslipidemia IIA in the presence of sarcopenia was observed (Figure 1b).
Dyslipidemia B showed to be related only to smoke. Interestingly, we observed in univariate analysis (Table 3) that both ex-smokers and still-smokers have a significant higher risk to experiment dyslipidemia IIB, with ex-smokers with an higher risk that stillsmokers (respectively, HR: 7.17 and HR: 4.09). In multivariate analyses, only the still-smoker groups have a significant increase of risk to have dyslipidemia IIB (Figure 1c).
Discussion
In the recent years, there has been increasing evidence of a relationship between obesity and metabolic dysfunction with poorer cancer prognosis, highlighting the importance of accurately assessing metabolic disturbance among cancer survivors [30-35]. Preventive measures, including dietary, counseling and tailored physical exercise should be initiated early in the course of survivorship; cancer treatment and lifestyle factors such as dietary intake, physical activity and smoking habits can cause direct endothelial damage and dysfunctions that contributes to insulin resistance and the development of the MetSyn; [36,37] in fact, the increase in caloric intake and consumption of refined sugars associated with physical inactivity and oncological therapies are the cause of the onset of abdominal obesity and insulin resistance triggering events of the MetSyn.
Inside into the aetiology of MetSyn in cancer survivors, which might differ from aetiology in general population, could lead to effective preventive and therapeutic measures; local treatments (surgery, and radiotherapy) and systemic cancer therapy (chemotherapy and hormone therapy) can cause changes in endocrine and metabolic functions that might contribute to the development of MetSyn. The cancer treatment induced MetSyn (CTIMetS) is multifactorial an differ between treatment type, cancer diagnosis and patients characteristics [38].
Two studies reported a higher prevalence of the MetSyn in patients with adult-onset malignant hematological disease who have received stem-cell transplantation preceded by chemotherapy alone or with whole body irradiation of 34% and 49% respectively [4,3].
Obesity also identified as excess malnutrition often causes sarcopenia and inflammatory processes with release of inflammatory mediators such as C-reactive protein and B-2 microglobulin whose blood levels can remain high long after the end of cancer treatments.
Sarcopenia is often detected in excess malnutrition in obesity or overweight. Obesity is often defined by the BMI that does not differentiate fat mass from the free fat mass [39]; this is relevant since it is the excess of fat mass no excess of body weight that represent a metabolic risk in cancer survivors, therefore it is more reliable the plicometric measurement that estimates the percentage of body fat. Muscle mass is a result of protein synthesis and degradation and sarcopenia is also characterized by the decrease in muscle cells, especially in the case of type II fibers. Excess malnutrition can cause progressive loss of FFM since obesity creates an anabolic resistance and therefore a reduced protein synthesis, moreover the obese subject has a greater protein degradation and insulin resistance which in turn has an anabolic effect [40,41].Obesity also causes accumulation of adipose tissue between muscle fibers, reducing muscle strength and increasing fatigue; the basal metabolism is lowered as a result of the loss of muscle mass triggering a cascade reaction that will tend to make you more and more fat [42-44].
The present study is the first that analyzed the onset of the metabolic syndrome in the two groups of adult long-term survivors with NHL and HL, correlating it with lifestyle and oncological treatments, in particular with the use of steroids that recent studies have identified as a possible cause of metabolic disorder and insulin resistance in cancer survivors.
Our results showed an excess malnutrition in the 64% of the lymphoma survivors and a higher prevalence of MetSyn in the NHL compared to the HL, associated with a significant loss of lean mass (sarcopenia) and higher levels of B-2 microglobulin in the NHL group.
Analysis of the questionnaires on lifestyle and eating habits showed an unbalanced diet with an excess of caloric intake compared to their measured real daily energy requirement (TDEE), very often rich in sugar and carbohydrates saturated fats and few fibers, only the 23% practiced physical activity regularly and the (25%) was a habitual smoker.
These date show how important it is to follow these patients from a nutritional and physical activity point of view. Currently, there are not special guidelines for treatment of the MetSyn in cancer survivors. The treatment of obesity in these patients should not be limited to providing a simple low-calorie diet but should aim at reducing excess fat mass and the cardiovascular and dysmetabolic risk factors associated with it. In these cancer survivors, it is essential that we can change the patient’s attitude towards nutrition and physical activities are the two main determinants of the energy balance. In conclusion, it would therefore be of fundamental importance to include the monitoring of the nutritional status of the long-term lymphoma survivors in clinical surveillance and provide nutritional advice and personalized food plans from the beginning of the treatments in order to avoid the onset of the syndrome and associated metabolic disorders.
Acknowledgements
The study was funded by the Italian Ministry of Health within the network of the Center for Disease Control (CMM 2014 grant B52F14000160001).
References
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006; 444: 881-7.
- Björntorp P, Rosmond R. The metabolic syndrome--a neuroendocrine disorder? Br J Nutr. 2000; 83: S49-57.
- Johannsson G, Bengtsson BA. Growth hormone and the metabolic syndrome. J Endocrinol Invest. 1999; 22: 41-6.
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord. 2000; 24: S50-5.
- Golub MS. The adrenal and the metabolic syndrome. Curr Hypertens Rep. 2001; 3: 117-20.
- Kauppila M, Koskinen P, Irjala K, Remes K, Viikari J. Long-term effects of allogeneic Bone Marrow Transplantation (BMT) on pituitary, gonad, thyroid and adrenal function in adults. Bone Marrow Transplant. 199; 22: 331-7.
- Schmiegelow M, Lassen S, Poulsen HS, Feldt-Rasmussen U, Schmiegelow K, Hertz H, et al. Growth hormone response to a growth hormone-releasing hormone stimulation test in a population-based study following cranial irradiation of childhood brain tumors. Horm Res. 2000; 54: 53-9.
- Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007; 49: 403-14.
- Luminari S, Cesaretti M, Rashid I, Mammi C, Montanini A, Barbolini E, et al. Incidence, clinical characteristics and survival of malignant lymphomas: a population-based study from a cancer registry in northern Italy. Hematol Oncol. 2007; 25: 189-97.
- Ciavarella S, Minoia C, Quinto AM, Oliva S, Carbonara S, Cormio C, et al. Improving Provision of Care for Long-term Survivors of Lymphoma. Clin Lymphoma Myeloma Leuk. 2017; 17: e1-e9
- Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, et al. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007; 8: 784-96.
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute. American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 2009. 120: 1640-5.
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Fernando Costa. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Circulation. 2005; 112: 2735-52.
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004; 4: 579-91.
- Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol. 2006; 169: 1505-22.
- Gurney JG, Ness KK, Sibley SD, O’Leary M, Dengel DR, Lee JM, et al. Metabolic syndrome and growth hormone deficiency in adult survivors of childhood acute lymphoblastic leukemia. Cancer. 2006; 107:1303-12.
- Ordovas JM. Genetic Links between diabetes mellitus and coronary atherosclerosis. Curr Atheroscler Rep. 2007; 9: p.204-10.
- de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010; 11: 193-203.
- Westerink NL, Nuver J, Lefrandt JD, Vrieling AH, Gietema JA, WalenkampAM. Cancer treatment induced metabolic syndrome: Improving outcome with lifestyle. Crit Rev Oncol Hematol. 2016; 108: 128-136.
- Talvensaari KK, Lanning M, Tapanainen P, Knip M. Long-term survivors of childhood cancer have an increased risk of manifesting the metabolic syndrome. J Clin Endocrinol Metab. 1996; 81: 3051-5.
- Jung HS, Myung SK, Kim BS, Seo HG. Metabolic syndrome in adult cancer survivors: a meta-analysis. Diabetes Res Clin Pract. 2012; 95: 275-82.
- Annaloro C, Usardi P, Airaghi L, Giunta V, Forti S, Orsatti A, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008; 41: 797-804.
- Strongman H, Brown A, Smeeth L, Bhaskaran K. Body mass index and Hodgkin’s lymphoma: UK population-based cohort study of 5.8 million individuals. Br J Cancer. 2019; 120: 768-770.
- Boyle T, Connors JM, Gascoyne RD, Berry BR, Sehn LH, Bashash M, et al. Physical activity, obesity and survival in diffuse large B-cell and follicular lymphoma cases. Br J Haematol. 2017; 178: 442-447.
- Minoia C, Ciavarella S, Lerario G, Daniele A, De Summa S, Napolitano M, et al. Improvable Lifestyle Factors in Lymphoma Survivors. Acta Haematol. 2018; 139: 235-237.
- Lohman T, Roche A, Martorell R. Antropometric Standardization reference manual. Human Kinetics Publishers. 1988; 2-80.
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014; 63: 2985-3023
- Durnin JV, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974; 32: 77-97.
- Costa NA, Marinho AD, Cançado LR. Nutritional requirements of the critically ill patient. Rev Bras Ter Intensiva. 2012 Sep; 24: 270-277
- Venables WN & Ripley BD. Modern Applied Statistics with S. Fourth Edition. Springer, New York. 2002 ISBN 0-387-95457-0
- Sheldon T. Cancer survival rates continue to rise in the Netherlands. BMJ. 2001; 322: 1509.
- Taskinen M, Saarinen-Pihkala UM, Hovi L, Lipsanen-Nyman M. Impaired glucose tolerance and dyslipidaemia as late effects after bone-marrow transplantation in childhood. Lancet. 2000; 356: 993-7.
- Didi M, Didcock E, Davies HA, Ogilvy-Stuart AL, Wales JK, Shalet SM. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J Pediatr. 1995; 127: 63-7.
- Malhotra J,Tonorezos ES, Rozenberg M, Vega GL, Sklar CA, Chou J, et al. Atherogenic low density lipoprotein phenotype in long-term survivors of childhood acute lymphoblastic leukemia. J Lipid Res. 2012; 53: 2747-54.
- Barbosa-Cortés L, López-Alarcón M, Mejía-Aranguré JM, Klünder-Klünder M, Del Carmen Rodríguez-Zepeda M, Rivera-Márquez H, et al. Adipokines, insulin resistance, and adiposityas a predictors of metabolicsyndrome in childsurvivors of lymphoma and acute lymphoblasticleukemia of a developing country. BMC Cancer. 2017; 17: 125.
- Morel S, Leahy J, Fournier M, Lamarche B, Garofalo C, Grimard G, et al. Lipid and lipoprotein abnormalities in acute lymphoblastic leukemia survivors. J Lipid Res. 2017; 58: 982-993.
- Ness KK, Hudson MM, Ginsberg JP, Nagarajan R, Kaste SC, Marina N, et al. Physical performance limitations in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009; 27: 2382-9.
- Herman DR, Ganz PA, Petersen L, Greendale GA. Obesity and cardiovascular risk factors in younger breast cancer survivors: The Cancer and Menopause Study (CAMS). Breast Cancer Res Treat. 2005; 93: 13-23.
- Kourti M, Tragiannidis A, Makedou A, Papageorgiou T, Rousso I, Athanassiadou F. Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J Pediatr Hematol Oncol. 2005; 27: 499-501.
- Cornier MA, Després JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation. 2011; 124: 1996-2019.
- Derbré F, Gratas-Delamarche A, Gómez-Cabrera MC, Viña J. Inactivityinducedoxidative stress: a centralrole in age-relatedsarcopenia? Eur J Sport Sci. 2014; 14: S98-108.
- Cheng-ChiehLin, Sharon LR Kardia, Chia-Ing Li, Chiu-ShongLiu, Ming-May Lai, Wen-Yuan Lin, et al. The relationship of high sensitivity C-reactive protein to percent body fat mass, body mass index, waist-to-hip ratio, and waist circumference in a Taiwanese population. BMC Public Health. 2010; 10: 579.
- Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008; 18: 388-95.
- Maffiuletti N, Jubeau M. Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol, 2007; 101: 51-59.
- Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015; 70: 57-62.
- Srikanthan P, Karlamangla A. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination. J Clin Endocrinol Metab, 2011; 96: 2898- 2903.