
Original Article
Ann Hematol Oncol. 2020; 7(1): 1283.
Real World Treatment Patterns and Comparative Effectiveness among Elderly Patients with Acute Myeloid Leukemia in the United States
Medeiros BC1 and Satram S2*
¹Department of Medicine, Stanford University, USA
²Department of Epidemiology, Q.D. Research, USA
*Corresponding author: Sacha Satram, Department of Epidemiology, Q.D. Research, Inc, 4120 Douglas Blvd., Ste 306436, Granite Bay, CA 95746, USA
Received: January 23, 2020; Accepted: February 13, 2020; Published: February 20, 2020
Abstract
An evaluation of treatment patterns and outcomes among 11,142 first primary Acute Myeloid Leukemia (AML) patients was conducted. There were 936 (8%) patients who were treated with azacitidine and/or decitabine (HMA), 153 (1%) received a cytarabine combination regimen (Intensive), 433 (4%) received another type of agent (Other), 3250 (29%) received an unidentified agent (Unknown) and 6,370 (57%) did not receive any treatment. There were 403 (8%) patients who underwent subsequent allogeneic Hematopoietic Stem Cell Transplantation (HSCT) therapy after initial chemotherapy. Overall, treatment rates increased over the study time-period from 36% in 2000 to 55% in 2013 (P ‹0.0001). Treated patients were more likely to be younger, male, and married, and were less likely to have secondary AML, poor performance, and comorbid conditions compared to untreated patients. Receipt of all types of antileukemic therapy showed significant mortality risk reductions compared with palliative care. HSCT was associated with a 40% mortality risk reduction versus chemotherapy only, and the survival benefit was more pronounced among patients ≤75 years. These findings provide a rationale to strongly consider antileukemic therapy rather than best supportive care in older patients who do not meet criteria for more intensive regimens.
Keywords: Acute myeloid leukemia; chemotherapy; hematopoietic stem cell transplantation, treatment, survival, elderly patients
Introduction
The incidence of Acute Myeloid Leukemia (AML) increases with age and over half of patients are diagnosed at age ≥65 years [1]. Although AML is a relatively rare disease accounting for just over 1% of adult cancer deaths in the United States [2], the incidence is expected to increase as the population ages. The prognosis of patients 65 years and older is very poor and worsens with advancing age as treatment efficacy and tolerability have been shown to deteriorate markedly in older adults. Without treatment, AML progresses rapidly and is fatal within a few weeks of diagnosis [3]. There is no optimal treatment strategies for older patients with AML so therapy is individualized based on medical fitness, age, cytogenetic/molecular testing, the potential benefits for short and long-term outcomes, and the potential risk of adverse events in the context of patient wishes and socio-economic support.
Conventional intensive chemotherapy remains the standard of care for younger, functionally fit patients. Older patients, however, are often not treated or given less intensive chemotherapy and have inferior clinical outcomes. In spite of these facts, the major causes of death from AML in older patients are from infection and hemorrhage related to disease-associated cytopenias [4]. Retrospective and population-based studies suggest that age is not a barrier to the beneficial effects of AML treatment in patients up to 80 years old [5,6] and others have reported high rates of response and improved survival with intensive therapy up to 90 years old [7,8]. In addition, allogeneic Hematopoietic Stem Cell Transplantation (HSCT) is often the only curative treatment modality for most AML patients older than 60 years [9-11]. Unfortunately, the majority of patients remain ineligible for HSCT.
Prospective studies may be subject to bias due to selection of younger and prognostically favorable patients receiving treatment. Randomized trials for AML patients 65 years and older are few, but have demonstrated a longer overall survival for intensively treated patients [12,13]. Prior population based analyses found that the use of chemotherapy has increased over time and was associated with a significant survival benefit compared to best supportive care [3,5]. However, there has been minimal improvement in survival as treatment strategies have not significantly changed for the past several decades ago. The goal of the study was to assess comparative effectiveness of existing therapeutic regimens, the patient characteristics associated with treatment receipt, and determine if treatment rates and survival continue to rise in a real-world population of elderly patients with AML.
Methods
Data sources
Data from the Surveillance, Epidemiology, and End Results (SEER) Medicare linked database was used for these analyses. Institutional Review Board (IRB) approval was waived because there are no personal identifiers in the SEER-Medicare database. The SEER-Medicare database is a collaborative effort of the National Cancer Institute (NCI), the SEER registries, and the Centers for Medicare & Medicaid Services and provides information on Medicare patients included in SEER, a nationally representative collection of 18 population-based registries of all incident cancers from diverse geographic areas [14]. The linked database includes all incident cancer patients reported to the SEER registries and cross-matched with a master file of enrollees in Medicare [15] with approximately 97% of persons 65 years or older eligible for Medicare. Inpatient care, skilled nursing care, home healthcare, and hospice care are covered services under Medicare Part A, while Part B reimburses for physician and outpatient care with about 95% of beneficiaries subscribing to Part B. The SEER-Medicare linkage used in this study include all Medicare eligible cancer patients reported to SEER through 2013 and their Medicare claims through 2015.
Study population
Patients were included if they were diagnosed with a first primary AML cancer from January 1, 2000 to December 31, 2013, ›66 years, and continuously enrolled in Medicare Parts A and B with no HMO coverage in the year prior to diagnosis (Supplementary Figure 1). Patients were excluded if their date of death was recorded prior to or the same month as diagnosis, if they were enrolled in a Health Maintenance Organization (HMO) at any time during the 12 months prior to diagnosis (because complete claims data were unavailable for these patients), and if they had two or more claims for chemotherapy prior to diagnosis.
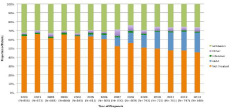
Figure 1: Therapy cohort by year of diagnosis.
Study variables
The SEER program registries routinely collect data on patient demographics (age, race/ethnicity, residence, and socioeconomic status [income and education per census tract]); primary tumor site, tumor morphology and stage at diagnosis; first course of treatment, and follow-up for vital status. AML diagnosis was based on the International Classification of Disease for Oncology (3rd edition, ICD-O-3) histology codes in the SEER data. Median annual household income at the census tract level, and percentage of adults aged 25 or older with at least some college education at the ZIP code level in the SEER data were used as a proxy for socioeconomic status.
Risk stratification in AML is based on cytogenetics and molecular abnormalities, which were not available in the SEER data. Prior Myelodysplastic Syndrome (MDS) or Myeloproliferative Neoplasm (MPN) that transforms into AML are also poor prognostic features, and occur more commonly among elderly patients. [16] In the absence of disease stage, prior MDS was used as a proxy for high risk patients and was identified using diagnosis codes in Medicare Parts A and B claims files prior to AML diagnosis. By design, patients with therapy-related AML (t-AML) were excluded based on the inclusion criteria of the study cohort. SEER also does not include measures of performance status, such as Eastern Cooperative Oncology Group. Instead, we used Medicare claims to identify several indictors of Poor Performance status (PPI) [17], including the use of oxygen and related respiratory therapy supplies, wheelchair and supplies, home health agency services, and skilled nursing facility services that occurred 12 months prior to AML cancer diagnosis.
To assess baseline comorbidity burden, we utilized the National Cancer Institute (NCI) comorbidity index [18] to identify the 15 noncancer comorbidities from the Charlson Comorbidity Index [19]. The index accounts for the number and seriousness of the conditions and a higher score indicates a greater burden of comorbid disease. Diagnosis and procedure codes were identified from Medicare claims one year prior to diagnosis and must appear on at least two different claims that are more than 30 days apart to ensure that “rule out” diagnoses are not counted as comorbid conditions.
Chemotherapy administration was identified using International Classification of Disease (9th revision), Clinical Modification (ICD-9- CM) diagnosis codes and procedural codes, and Healthcare Common Procedural Coding System (HCPCS) “J” codes were used to identify the specific drug administered [20]. The absence of these claims indicated lack of treatment. The first chemotherapy claim within three months from diagnosis indicated the start of therapy. Patients were classified into treatment groups based on all chemotherapy administered during the first 60 days after treatment initiation. Chemotherapy agent definition was not possible in approximately 70% of patients who received therapy because chemotherapy was administered during inpatient admissions, which are paid based on ICD-9 diagnosis or procedures codes only and not chemotherapy codes. Medicare claims files were also searched for ICD-9-CM and HCPCS codes to identify patients undergoing allogeneic Hematopoietic Stem Cell Transplantation (HSCT) anytime during follow-up.
Overall survival was measured from diagnosis date to date of death. The date of death was assigned by using the Medicare date or SEER date of death if Medicare date was missing. All other patients were assumed to be alive at the end of the follow-up period (December 31, 2015).
Statistical analysis
All statistical analyses were performed using SAS software, version 9.1.3 (SAS Institute Inc., Cary, North Carolina). Demographic and clinical characteristics were summarized descriptively by treatment status (treated vs. not treated) and treatment type. Chi-square test for categorical variables and ANOVA or t-test for continuous variables determined differences between groups. We considered a p-value ‹.05 to be statistically significant.
In the overall survival analyses we made comparisons between the treated and not treated patients; between treated patients receiving HSCT and those who did not; and between those receiving low dose therapy with azacitidine or decitabine (HMA Therapy), aggressive induction therapy with a cytarabine combination regimen (Intensive Therapy), other type of agents including hydroxyurea, cytarabine only, gemtuzumab, arsenic trioxide, lenalidomide, clofarabine, rituximab, or vincristine (Other Therapy), an unidentified agent (Unknown Therapy) and those not receiving treatment (Not Treated). Kaplan- Meier survival curves and corresponding log-rank tests examined unadjusted overall survival by treatment group. Since timing of treatment initiation differed between patients, the relationship between treatment and survival was evaluated using a Cox regression model with treatment as a time-dependent factor. In the time-varying Cox model, all patients belong to the “not treated” group and only switched to the “treated” group at the time of treatment receipt. Other confounders included in the Cox model were selected a priori from baseline demographic and clinical characteristics.
To assess the risk of early death (30-day mortality and 60- day mortality) a Cox regression model with treatment as a timedependent factor was constructed. The “treated” group was limited to patients who received treatment within 30 days after diagnosis to minimize the introduction of immortal time bias in the analysis (period of follow-up time during which death cannot occur) [21].
As a sensitivity exercise for the comparison between HMA Therapy, Intensive Therapy, Other Therapy, Unknown Therapy and No Treatment, we also conducted a propensity score-matched survival analysis. Multinomial logistic regression was used to calculate a propensity score the conditional probability that each patient would be assigned to a specific treatment group given that patient’s pretreatment variables [22,23]. Pairwise matching was conducted where each patient receiving a specific therapy (HMA, Intensive, Other, or Unknown) was matched to one untreated patient. Matching variables were age, sex, race, marital status, education, geographic region, year diagnosed, prior MDS, poor performance indicators, and comorbidity score. Matched survival analysis was completed using the Cox proportional hazards regression model, stratifying on the matched pair.
In the survival models, follow-up was calculated beginning on the date of diagnosis up until the first occurrence of a censoring event: date of death, the last date for which Medicare claims are available, or the end of the follow-up period (December 31, 2015).
Results
Treatment trends over time
Of the 11,142 patients included in the study, 4,772 (43%) patients received anti-leukemic treatment with chemotherapy within 3 months of diagnosis and 6,370 (57%) patients did not receive any antileukemic treatment. Treatment rates increased over the study time period from 36% in 2000 to 55% in 2013 (p ‹0.0001). Of those initiating treatment, 936 (8%) could be confirmed to have been treated with azacitidine and/or decitabine (HMA), 153 (1%) were confirmed to receive treatment with a cytarabine combination regimen (Intensive), 433 (4%) received another type of agent (Other) and 3,250 (29%) received an unidentified agent (Unknown). From 2005 to 2013, use of HMA therapy significantly increased from 2% to 22%, while during the same time period, the proportion of patients who did not receive treatment significantly decreased from 63% to 45% (Figure 1). Rates of Intensive therapy remained fairly consistent throughout the entire study period. Of 4,772 patients initiating treatment, 403 (8%) eventually underwent HSCT therapy after chemotherapy and 4,369 (92%) did not. Rates of HSCT also increased over the study time period from 7% in 2000 to 12% in 2012 (p=0.0033).
Outcomes according to treatment status
The distribution of patient characteristics by treatment status is shown on (Table 1). The mean age at diagnosis was 75 years for treated patients and 80 years for untreated patients (P ‹0.0001). Forty-three percent of treated patients were over the age of 75 years at diagnosis compared with 74% of untreated patients. Treated patients were more likely to be male (54% vs. 50%), be married (60% vs. 46%), to have a lower incidence of prior MDS (14% vs. 19%), were less likely to have PPIs (8% vs. 19%), and had a lower comorbidity burden (54% vs. 43% with a comorbidity score of 0) compared with untreated patients.
Total
(N = 11142)Treated
(N = 4772)Not Treated
(N = 6370)
HMA
N = 936Intensive
N =153Other
N = 433Unknown
N = 3250n
%
%
%
p a
%
%
%
%
p b
Age at Diagnosis
66-70
2008
28.8
10
18
< 0.0001
15.7
41.8
20.8
33
< 0.0001
71-75
2390
28.5
16.2
21.5
23
29.4
23.3
30.7
76-80
2586
23.2
23.2
23.2
27.2
16.3
24.2
22.2
81-85
2243
13.2
25.3
20.1
24.1
12.4c
17.3
9.8
>86
1915
6.3
25.3
17.2
9.9
14.3
4.3
Sex
Male
5810
54.4
50.4
52.1
< 0.0001
58
60.8
48.5
53.9
<0.0001
Female
5332
45.6
49.6
47.9
42
39.2
51.5
46.1
Race/ethnicity
White
9673
86.9
86.7
86.8
0.0301
88.6
88.2
85.9
86.6
0.0795
Black
681
5.5
6.5
6.1
4.4
11.7c
4.6
6
Other/Unknown
788
7.5
6.7
7.1
7.1
9.5
7.4
Prior MDS1
No
9243
85.8
80.8
83
<0.0001
84.3
92.8
82.9
86.3
<0.0001
Yes
1899
14.2
19.2
17
15.7
7.2
17.1
13.7
PPI2
No
9573
92.4
81.1
85.9
<0.0001
89.5
100.0c
89.6
93.4
<0.0001
Yes
1569
7.6
18.9
14.1
10.5
10.4
6.6
NCI Co-morbidity Score
0
5303
53.5
43.2
47.6
<0.0001
44.8
54.9
50.8
56.3
<0.0001
1
2854
25.9
25.4
25.6
26.7
28.8
26.3
25.4
2
1473
10.9
14.9
13.2
13.4
16.4c
12.7
10
=3
1512
9.7
16.5
13.6
15.2
10.2
8.3
Marital Status
Married
5796
60.4
45.7
52
<0.0001
59.4
71.9
54.7
60.9
<0.0001
Single
774
7.2
6.8
6.9
7.5
9.8c
5.5
7.4
Separated/Divorced
750
6.6
6.8
6.7
5.9
6.2
7.1
Widowed
3273
21.3
35.4
29.4
22.8
18.3c
28.4
20.3
Unknown
549
4.4
5.3
4.9
4.5
5.1
4.4
%of adults with some college education
0-50
3354
29.7
30.4
30.1
0.6026
26.8
28.1
31.6
30.3
0.2363
51-100
7582
68.4
67.8
68
70.6
71.9c
68.3c
67.9
Unknown
206
1.9
1.8
1.8
2.6
1.8
Median Income Quartiles
1-Low
2734
23.7
25.2
24.5
0.0051
20.4
24.2
28.9
23.9
0.0085
2
2734
24.5
24.6
24.5
25.1
23.5
23.8
24.4
3
2735
23.8
25.1
24.5
24.5
28.1
21
23.8
4-High
2733
26.1
23.3
24.5
27.5
24.2c
26.3c
26
Unknown
206
1.9
1.8
1.8
2.6
1.8
Geographic region
Midwest
1124
11
9.4
10.1
0.0457
9
11.1
6.7
12.1
<0.0001
Northeast
680
6.3
5.9
6.1
6.2
9.2
6.7
6.2
South
4131
36.6
37.4
37.1
34.4
29.4
34.9
37.8
West
5207
46.1
47.2
46.7
50.4
50.3
51.7
43.9
Abbreviations: NCI: National Cancer Institute; MDS: Prior Myelodysplastic Syndrome; PPI: Poor Performance Indicators.
ap-value Treated vs. Not Treated
bp-value Not Treated vs. HMA vs. Intensive vs. Other vs. Unknown
cCells with counts of less than 11 are combines in compliance with the National Cancer Institute data use agreement for small cell sizes.
1Patients with a prior Myelodysplastic Syndrome (MDS) or myeloproliferative disease was identified from Medicare claims and was used as a proxy for high risk patients in the absence of disease stage.
2Poor Performance Indicators (PPI) were identified from Medicare claims and include the use of oxygen and related respiratory therapy supplies, wheelchair and supplies, home health agency services, and skilled nursing facility services that occurred 12 months prior to AML diagnosis.
Table 1: Baseline patient characteristics by treatment status and treatment type.
All Treated
≤75 year olds
>75 year olds
HSCT
N = 403No HSCT
N = 4369pa
HSCT
N = 329No HSCT
N = 2404pb
HSCT
N = 74No HSCT
N = 1965pc
Age at Diagnosis
%
%
%
%
%
%
66-70
55.1
26.4
<0.0001
67.5
47.9
<0.0001
0.8045
71-75
26.6
28.7
32.5
52.1
76-80
10.4
24.4
56.8
54.2
81-85
7.9d
13.9
43.3d
30.9
>86
6.7
14.9
Sex
Male
62.5
53.7
0.0007
61.7
54.6
0.0153
66.2
52.6
0.0209
Female
37.5
46.3
38.3
45.4
33.8
47.4
Race/ethnicity
White
88.3
86.8
0.3577
88.4
85.8
0.1332
100.0d
88
0.991
Black
4
5.7
4
6.9
4.2
Other/Unknown
7.7
7.5
7.6
7.3
7.7
Prior MDS1
No
89.1
85.5
0.0507
89.4
86.8
0.189
100.0d
84
0.3773
Yes
10.9
14.5
10.6
13.2
16
PPI2
No
96
92
0.0038
100.0d
94
0.016
100.0d
89.6
0.7982
Yes
4
8
6
10.4
NCI Co-morbidity Score
0
59.3
53
0.0571
63.2
55.1
0.0217
41.9
50.4
0.3105
1
23.3
26.1
22.8
26.3
25.7
25.8
2
7.9
11.2
6.4
10.4
14.9
12.2
=3
9.4
9.7
7.6
8.2
17.6
11.6
Marital Status
Single
7.4
7.2
0.0033
7.3
8.5
0.4024
59.5d
5.5
0.7146
Married
65.8
59.9
69
65
53.6
Separated/Divorced
8.7
6.5
9.1
8
40.5d
4.5
Widowed
14.1
22
10.9
13.9
31.9
Unknown
4
4.5
3.6
4.5
4.5
%of adults with some college education
0-50
24.8
30.1
0.0748
24.3
32.2
0.013
27
27.5
0.9402
51-100
75.1d
68
75.7d
65.8
73.0d
70.6
Unknown
1.9
2
1.9
Median Income Quartiles
1-Low
19.9
24
0.0386
20.1
25.8
0.0004
18.9
21.9
0.4627
2
21.6
24.7
19.5
25.7
31.1
23.5
3
27
23.5
27.7
23.5
24.3
23.6
4-High
29.8
25.8
31
23
24.3
29.1
Geographic region
Midwest
10.2
11
0.0027
12.7d
10.8
0.0005
13.5d
11.3
0.8496
Northeast
2.7
6.6
7.7
5.4
South
34.2
36.8
33.4
36.3
37.8
37.5
West
52.9
45.5
53.8
45.3
48.6
45.8
Abbreviations: AD: Azacitidine +Decitabine; CA: Cytarabine +Anthracycline; HSCT: Allogeneic Hematopoietic Stem Cell Transplantation; NCI: National Cancer Institute; MDS: Prior Myelodysplastic Syndrome; PPI: Poor Performance Indicators.
ap-value HSCT vs. No HSCT among all chemotherapy treated patients
bp-value HSCT vs. No HSCT among patients ≤75 year olds
cp-value HSCT vs. No HSCT among patients >75 year olds
dCells with counts of less than 11 are combined in compliance with the National Cancer Institute data use agreement for small cell sizes
1Patients with a prior Myelodysplastic Syndrome (MDS) or myeloproliferative disease was identified from Medicare claims and was used as a proxy for high risk patients in the absence of disease stage.
2Poor Performance Indicators (PPI) were identified from Medicare claims and include the use of oxygen and related respiratory therapy supplies, wheelchair and supplies, home health agency services, and skilled nursing facility services that occurred 12 months prior to AML diagnosis
Table 2: Baseline patient characteristics by HSCT status.
The median unadjusted overall survival was 2.13 months (95% CI: 0.93-7.07) for the overall population and was longer for treated patients (5.3 months, 95% CI: 5.0-5.8) compared with untreated patients (1.6 months, 95% CI 1.6-1.7; log-rank P ‹0.0001). In multivariate survival analysis (Table 3), treated patients exhibited a 14% lower risk of death compared with untreated patients (HR 0.86; 95% CI 0.81–0.91). Advanced age at diagnosis, higher comorbidity score, presence of PPIs, and being unmarried were significantly associated with higher mortality risk.
Covariates
Overall Survivala
30-day mortalityab
60-day mortalityab
N = 11,142
N = 11,142
N = 11,142
N
HR
95% CI
HR
95% CI
HR
95% CI
Treatment Status
Not-treated (ref)
6370
Treated
4772
0.86
0.81-0.91
0.333
0.29-0.38
0.593
0.55-0.64
Age at Diagnosis
66-70 (ref)
2008
71-75
2390
1.253
1.16-1.35
1.143
0.98-1.34
1.283
1.15-1.43
76-80
2586
1.502
1.39-1.62
1.178
1.01-1.37
1.404
1.26-1.56
81-85
2243
1.714
1.58-1.86
1.415
1.22-1.65
1.612
1.45-1.79
>86
1915
2.097
1.92-2.30
1.772
1.52-2.06
2.129
1.91-2.37
Sex
Female (ref)
5332
Male
5810
1.019
0.97-1.07
0.975
0.89-1.07
0.993
0.93-1.06
Race/ethnicity
White (ref)
9673
Black
681
0.84
0.75-0.94
0.777
0.64-0.94
0.874
0.77-0.99
Other/Unknown
788
0.85
0.77-0.94
0.874
0.74-1.04
0.834
0.74-0.94
Marital Status
Married (ref)
5796
Single
774
1.237
1.12-1.36
1.235
1.05-1.45
1.29
1.15-1.45
Separated/Divorced
750
1.201
1.09-1.32
1.31
1.11-1.54
1.218
1.08-1.37
Widowed
3273
1.176
1.11-1.25
1.135
1.02-1.26
1.158
1.08-1.25
Unknown
549
0.997
0.89-1.12
1.108
0.92-1.34
1.063
0.93-1.22
Prior MDS1
No (ref)
9243
Yes
1899
0.998
0.94-1.07
1.01
0.91-1.12
0.972
0.90-1.05
PPI2
No (ref)
9573
Yes
1569
1.205
1.12-1.30
1.398
1.26-1.56
1.306
1.21-1.41
NCI Co-morbidity Score
0 (ref)
5303
1
2854
1.15
1.08-1.22
1.069
0.96-1.19
1.106
1.03-1.19
2
1473
1.227
1.14-1.33
1.233
1.09-1.39
1.341
1.23-1.46
=3
1512
1.324
1.22-1.44
1.352
1.20-1.53
1.4
1.28-1.53
Abbreviations: NCI: National Cancer Institute; MDS: Prior Myelodysplastic Syndrome; PPI: Poor Performance Indicators.
1Patients with a prior Myelodysplastic Syndrome (MDS) or myeloproliferative disease was identified from Medicare claims and was used as a proxy for high risk patients in the absence of disease stage.
2Poor Performance Indicators (PPI) were identified from Medicare claims and include the use of oxygen and related respiratory therapy supplies, wheelchair and supplies, home health agency services, and skilled nursing facility services that occurred 12 months prior to AML diagnosis.
aModel also includes geographic region, income and year of diagnosis.
bTreated group restricted to patients who received treatment within 30 days after diagnosis.
Table 3: Adjusted overall survival and risk of early death by treatment status.
The multivariate analysis of factors predicting early death is shown in (Table 3). There were 2359 (21%) of patients who died within 30 days of diagnosis and 4743 (43%) that died within 60 days of diagnosis. Stratifying by treatment status, 263 (2%) treated patients and 2096 (19%) untreated patients died within 30 days of diagnosis. Treated patients had a 67% lower likelihood of early death within 30 days of diagnosis and a 41% lower likelihood of early death within 60 days of diagnosis compared to the untreated cohort. Other factors associated with increased risk of early death include older age, unmarried, higher comorbidity burden and presence of poor performance indicators.
Outcomes according to treatment modality
There were similarities in demographic and clinical characteristics between patients receiving Intensive and Unknown therapies and between patients receiving HMA, Other therapies, and those who were untreated (Table 1). Patients receiving Intensive chemotherapy and Unknown therapy were younger, had lower comorbidity burden, and were less likely to have prior Myelodysplastic Syndromes (MDS) or PPIs.
Figure 2 shows the median unadjusted overall survival was longest for patients receiving Intensive therapy (16.7 months), followed by HMA therapy (7.3 months), Unknown therapy (4.5 months), Other therapy (3.5 months), and was shortest for untreated patients (1.6 months; log-rank p ‹0.0001). After adjusting for age, sex, race, MDS, PPIs, comorbidity score, marital status, income, education, geographic region, and year of diagnosis in the survival model, receipt of all types of antileukemic therapy demonstrated significantly improved survival compared with palliative care or no treatment (Table 4). Compared to the untreated group, patients who received Intensive therapy had the largest statistically significant reduction in overall mortality risk (68%), followed by a 53% mortality risk reduction among patients who received HMA therapy, 51% among patients who received Other Therapy and 34% among those received an Unknown therapy type. The propensity score-matched survival model confirmed the significant improvement in overall survival among all treatment groups compared to no treatment. Intensive and HMA therapy groups have overlapping confidence intervals, but both groups exhibited statistically significant lower mortality risks compared to Other therapy and Unknown therapy groups.
Covariates
N
Multivariate Cox
RegressionaPropensity-Weighted
Cox RegressionbSubpopulation
HR
95% CI
HR
95% CI
Not Treated (ref)
6370
HMA Therapy
936
0.471
0.43-0.52
0.529
0.49-0.57
Intensive Therapy
153
0.317
0.25-0.40
0.437
0.37-0.51
Other Therapy
433
0.49
0.43-0.56
0.692
0.63-0.77
Unknown Therapy
3250
0.66
0.62-0.70
0.742
0.71-0.77
Age at Diagnosis
66-70 (ref)
2008
71-75
2390
1.228
1.14-1.33
76-80
2586
1.418
1.31-1.53
81-85
2243
1.546
1.42-1.68
>86
1915
1.793
1.64-1.97
Sex
Female (ref)
5332
Male
5810
1.018
0.97-1.07
Race/ethnicity
White (ref)
9673
Black
681
0.814
0.73-0.91
Other/Unknown
788
0.837
0.76-0.92
Marital Status
Married (ref)
5796
Single
774
1.226
1.11-1.35
Separated/Divorced
750
1.144
1.04-1.26
Widowed
3273
1.15
1.08-1.22
Unknown
549
0.963
0.86-1.08
Prior MDS1
No (ref)
9243
Yes
1899
0.977
0.92-1.04
PPI2
No (ref)
9573
Yes
1569
1.146
1.06-1.24
NCI Co-morbidity Score
0 (ref)
5303
1
2854
1.158
1.09-1.23
2
1473
1.228
1.14-1.33
=3
1512
1.31
1.21-1.42
Abbreviations: NCI: National Cancer Institute; MDS: Prior Myelodysplastic Syndrome; PPI: Poor Performance Indicators.
1Patients with a prior Myelodysplastic Syndrome (MDS) or myeloproliferative disease was identified from Medicare claims and was used as a proxy for high risk patients in the absence of disease stage.
2Poor Performance Indicators (PPI) were identified from Medicare claims and include the use of oxygen and related respiratory therapy supplies, wheelchair and supplies, home health agency services, and skilled nursing facility services that occurred 12 months prior to AML diagnosis.
aModel also includes geographic region, income and year of diagnosis.
bPropensity score weighted for age, sex, race, prior MDS, PPI, NCI comorbidity score, geographic region, income and year of diagnosis.
Table 4: Adjusted overall survival by treatment type.
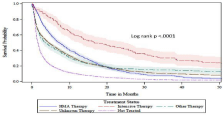
Figure 2: Unadjusted overall survival by treatment type.
Effect of allogeneic stem cell transplantation on survival
Of the 403 patients who underwent HSCT, the majority (82%) were aged ≤75 years (Table 2). Patients who underwent HSCT were younger at diagnosis, more likely to be male, more likely to be married, less likely to have prior MDS or PPIs, and had a lower comorbidity score versus patients treated with chemotherapy only. In a subset analysis stratified by age, the relationships observed between patient characteristics and HSCT receipt persisted in the younger cohort of patients (≤75 years) but not in the older cohort (›75 years).
Figures 3a-c shows the unadjusted median overall survival was higher for the HSCT group (14.2 months) versus the non-HSCT group (4.8 months; log-rank P ‹0.0001). This relationship was corroborated in the younger aged cohort ≤75 years where the unadjusted median overall survival was higher for the HSCT group (17.9 months) versus the non-HSCT group (6.6 months; log-rank P ‹0.0001), but not in the older cohort aged ›75 where the median overall survival was 4.5 months for the HSCT group vs. 3.3 months in the non-HSCT group (log rank p=0.3774).
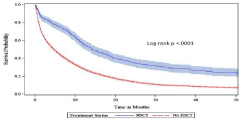
Figure 3a: Kaplan-Meier Curve of Overall Survival by HSCT.
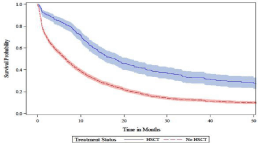
Figure 3b: Patients aged ≤75 years.
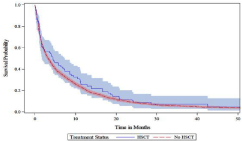
Figure 3c: Patients aged ›75 years.
In multivariate survival analysis (Table 5), patients who underwent HSCT had a 40% lower risk of death versus those who did not undergo HSCT (HR 0.60; 95% CI 0.53–0.67). Advanced age, male sex, higher comorbidity score, prior MDS, and PPIs were significantly associated with higher risk of mortality. Stratifying by age, the survival benefit with HSCT was only demonstrated in the younger cohort aged ≤75 years (HR 0.53; 95% CI 0.46–0.60) and no difference in mortality risk was noted in the older cohort aged ›75 years (HR 0.85; 95% CI 0.67–1.08).
Treateda
N = 4772≤75yearsa
N = 2733>75yearsa
N = 2039HSCT
N
HR
95% CI
HR
95% CI
HR
95% CI
No (ref)
4369
Yes
403
0.598
0.53-0.67
0.526
0.46-0.60
0.852
0.67-1.08
Age at Diagnosis
66-70 (ref)
1374
71-75
1359
1.204
1.11-1.30
76-80
1108
1.45
1.33-1.58
81-85
630
1.704
1.54-1.89
>86
301
2.131
1.86-2.44
Sex
Female (ref)
2174
Male
2598
1.111
1.04-1.18
1.11
1.02-1.21
1.104
1.00-1.22
Race/ethnicity
White (ref)
4149
Black
264
0.891
0.78-1.02
0.936
0.79-1.11
0.797
0.63-1.02
Other/Unknown
359
0.919
0.82-1.03
0.926
0.79-1.08
0.902
0.76-1.07
Marital Status
Married (ref)
2882
Single
343
1.184
1.05-1.33
1.148
0.99-1.34
1.12
0.92-1.37
Separated/Divorced
317
1.285
1.14-1.45
1.176
1.01-1.36
1.473
1.19-1.83
Widowed
1018
1.197
1.10-1.30
1.263
1.12-1.43
1.235
1.11-1.38
Unknown
212
1.085
0.94-1.26
0.979
0.80-1.19
1.26
1.01-1.57
Prior MDS1
No (ref)
4096
Yes
676
1.188
1.09-1.29
1.188
1.06-1.34
1.195
1.06-1.35
PPI2
No (ref)
4408
Yes
364
1.156
1.03-1.30
1.206
1.00-1.45
1.162
0.99-1.36
NCI Co-morbidity Score
0 (ref)
2553
1
1234
1.144
1.06-1.23
1.168
1.06-1.29
1.122
1.00-1.25
2
522
1.189
1.07-1.32
1.201
1.04-1.38
1.172
1.01-1.36
=3
463
1.408
1.26-1.57
1.448
1.24-1.69
1.386
1.18-1.62
Abbreviations: NCI: National Cancer Institute; MDS: Prior Myelodysplastic Syndrome; PPI: Poor Performance Indicators.
1Patients with a Prior Myelodysplastic Syndrome (MDS) or myeloproliferative disease was identified from Medicare claims and was used as a proxy for high risk patients in the absence of disease stage.
2Poor Performance Indicators (PPI) were identified from Medicare claims and include the use of oxygen and related respiratory therapy supplies, wheelchair and supplies, home health agency services, and skilled nursing facility services that occurred 12 months prior to AML diagnosis.
aModel also includes geographic region, income and year of diagnosis.
Table 5: Adjusted overall survival among treated patients with and without HSCT.
Discussion
We confirmed the observations from our original work [3] that the use anti-leukemic chemotherapy among elderly AML patients in real-world practice continues to increase. Most of this increase was driven by increased use of HMAs between 2005 and 2013 (from 2% to 22%), with a proportional reduction of untreated patients. Despite similar baseline characteristics in patients in the HMA, Other Therapy and Not Treated groups, treatment with HMAs and Other therapies were associated with statistically significant improvement in survival. Findings from a prior comparative observational study and a randomized trial support findings that azacitidine was associated with better survival compared to conventional care with intensive chemotherapy and BSC among poor-risk patients ages 65 years and older with newly diagnosed AML [24,25]. Even with increasing therapy use at the time of this analysis, nearly half (45%) of subjects still did not receive treatment, and the rate of no treatment was even more pronounced (74%) among patients ›75 years at diagnosis. These observations highlight the need for novel therapies for AML patients with advancing age and co-morbidities, such as the recent FDA approval of BCL-2 inhibitors in combination with HMAs or low-dose intensive therapy for treatment of newly diagnosed AML patients who are ≥75 years of age, or had comorbidities that precluded the use of intensive induction chemotherapy due to comorbidities [26].
The current study went further to evaluate age-specific treatment patterns and outcomes surrounding use of HSCT. In the current study, 8% of AML patients that received antileukemic therapy subsequently underwent subsequent HSCT therapy with the majority (82%) being between 66-75 years of age. Rates of HSCT increased by 5% over the 12-year study time period. This is consistent with previous reports of a steady increase in HSCT utilization among patients 70 years and older [27]. In addition, others have also shown that advancing age may be associated with worse post-HSCT outcomes in patients with AML [28]. In the multivariate survival analysis, HSCT was associated with a 40% reduction in mortality risk versus patients receiving chemotherapy only; however, this survival benefit was limited to patients aged 66-75 years. In spite of these findings, our observations show an important survival benefit with HSCT in select older patients with AML.
Strengths & Limitations
Real-world observational studies are considered inferior to RCT’s, however the information generated are complementary to findings from RCTs as clinical trial participants are subject to more controlled environments and are potentially not representative of individuals in the real-world. Therefore, there SEER-Medicare database is an invaluable tool to examine therapeutic strategies in actual practice settings among patients who have been historically underrepresented in clinical trials. With over 11,000 patients, it is one of the largest samples of AML patients with diverse geographic representation in the US. The SEER program is considered the gold standard in cancer surveillance and the linkage of claims based data from Medicare reduces the likelihood of treatment misclassification. However, with insurance claims data, we cannot determine physician’s intent, equal access to medical care, or be able to capture treatments paid out of pocket. Furthermore, for a significant amount (68%) of the treated cohort, we were unable to define the type of chemotherapy received due to the highly toxic nature of conventional chemotherapy treatments [29] for AML which requires urgent initiation of therapy in the inpatient setting. Inpatient admissions are paid out my Medicare based on ICD-9 diagnosis or procedures codes only and not on the chemotherapy J code administered. For a comprehensive examination of the comparative effectiveness of all anti-leukemic therapy, we included the patients who received Unknown therapy as a separate group. Finally, we cannot exclude the possibility of residual confounding in examining prognostic factors due to the absence of cytogenetic risk status and performance status in the dataset. We used Medicare claims to identify patients with a prior MDS and used this as a marker of disease severity, and also used Medicare claims to identify several indictors of poor performance. Performance status and disease severity influence clinicians’ decisions to treat or the specific regimen to administer and these proxy variables may not adequately assess risk status or physical fitness for all patients in our study.
In conclusion, the findings from this study provide a rationale to strongly consider therapy rather than best supportive care in older patients who do not fit the criteria for more intensive regimens. While there are challenges in treating elderly patients with AML, these results indicate that age alone should not disqualify a patient from receiving intensive induction chemotherapy if they have favorable prognostic characteristics. Following the recent approval of BCL-2 inhibitors in combination with HMAs or low-dose intensive therapy, our results will serve as an important historical control for incidence of treated patients and their survival outcomes in this fragile cohort of AML patients.
Acknowledgements
Funding for this study was provided by Celgene, Inc. The authors would like to thank Faiyaz Momin for statistical analysis and Monika Parisi for input on the study design. This study used the linked SEER–Medicare database. We acknowledge the efforts of the Applied Research Program, NCI (Bethesda, MD), the Office of Information Services and the Office of Strategic Planning, Health Care Financing Administration (Baltimore, MD), Information Management Services, Inc. (Silver Spring, MD), and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors.
References
- Bethesda MD. SEER Stat Fact Sheets: Acute Myeloid Leukemia.
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60: 277-300.
- Medeiros BC, Satram HS, Hurst D, Hoang KQ, Momin F and Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015; 94: 1127- 1138.
- Klepin HD. Myelodysplastic Syndromes and Acute Myeloid Leukemia in the Elderly. Clin Geriatr Med. 2016; 32: 155-173.
- Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012; 97: 1916-1924.
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Mollgard L and Stockelberg D, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009; 113: 4179-4187.
- Wetzler M, Mrozek K, Kohlschmidt J, Dombret H, Dohner H and Pilorge S, et al: Intensive induction is effective in selected octogenarian acute myeloid leukemia patients: prognostic significance of karyotype and selected molecular markers used in the European Leukemia Net classification. Haematologica. 2014; 99: 308-313.
- Harb AJ, Tan W, Wilding GE, Ford L, Sait SN and Block AW, et al. Treating octogenarian and nonagenarian acute myeloid leukemia patients--predictive prognostic models. Cancer. 2009; 115: 2472-2481.
- Krenger W, Blazar BR, Hollander GA. Thymic T-cell development in allogeneic stem cell transplantation. Blood. 2011; 117: 6768-6776.
- Herr AL, Labopin M, Blaise D, Milpied N, Potter M and Michallet M, et al. HLAidentical sibling allogeneic peripheral blood stem cell transplantation with reduced intensity conditioning compared to autologous peripheral blood stem cell transplantation for elderly patients with de novo acute myeloid leukemia. Leukemia. 2007; 21: 129-135.
- Storb R. Can reduced-intensity allogeneic transplantation cure older adults with AML? Best Pract Res Clin Haematol. 2007; 20: 85-90.
- Vey N, Coso D, Bardou VJ, Stoppa AM, Braud AC and Bouabdallah R, et al. The benefit of induction chemotherapy in patients age › or = 75 years. Cancer. 2004; 101: 325-331.
- Mori M, Ohta M, Miyata A, Higashihara M, Oshimi K and Kimura H, et al. Treatment of acute myeloid leukemia patients aged more than 75 years: results of the E-AML-01 trial of the Japanese Elderly Leukemia and Lymphoma Study Group (JELLSG). Leuk Lymphoma. 2006; 47: 2062-2069.
- Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002; 40: 3-18.
- Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993; 31: 732-748.
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ and Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009; 114: 937-951.
- Davidoff AJ, Tang M, Seal B, Edelman MJ. Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010; 28: 2191-2197.
- Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007; 17: 584- 590.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40: 373-383.
- Warren JL, Harlan LC, Fahey A, Virnig BA, Freeman JL and Klabunde CN, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002; 40: 55-61.
- Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008; 167: 492-499.
- Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM and Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006; 163: 262-270.
- Rosenbaum PR, Rubin DB. The Central Rose of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983; 70: 41-55.
- Heiblig M, Elhamri M, Tigaud I, Plesa A, Barraco F and Labussiere H, et al. Treatment with Low-Dose Cytarabine in Elderly Patients (Age 70 Years or Older) with Acute Myeloid Leukemia: A Single Institution Experience. Mediterr J Hematol Infect Dis. 2016; 8: e2016009.
- Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D and Jang JH, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015; 126: 291-299.
- DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M and Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019; 133: 7-17.
- Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X and Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood. 2017; 130: 1156-1164.
- Aoki J, Kanamori H, Tanaka M, Yamasaki S, Fukuda T and Ogawa H, et al. Impact of age on outcomes of allogeneic hematopoietic stem cell transplantation with reduced intensity conditioning in elderly patients with acute myeloid leukemia. Am J Hematol. 2016; 91: 302-307.
- Williams JP, Handler HL. Antibody-targeted chemotherapy for the treatment of relapsed acute myeloid leukemia. Am J Manag Care. 2000; 6: 975-985.