
Case Report
Ann Hematol Oncol. 2020; 7(2): 1287.
Early Responses Can Be Safely Achieved with Polatuzumab and Rituximab without Bendamustine in Multiply Relapsed and Refractory Diffuse Large B Cell Lymphoma
Talaulikar D1,2* and Garg A3
¹Department of Hematology, Canberra Hospital, Australia
²Australian National University Medical School, College of Biology, Medicine and Environment, Australian National University, Australia
³Department of Radiology, Canberra Hospital, Australia
*Corresponding author: Dipti Talaulikar, Department of Hematology, Canberra Hospital, Yamba Drive, Garran, Australian Capital Territory 2605, Australia
Received: February 04, 2020; Accepted: March 09, 2020; Published: March 16, 2020
Abstract
Polatuzumab, Bendamustine and Rituximab has been reported to improve outcome in relapsed and refractory cases of Diffuse Large B cell Lymphoma (DLBCL). The contribution of Bendamustine, which causes T cell lymphocytopenia to the treatment regimen is unknown. We report a 75 year old patient with 5 prior lines of treatment, who developed a rapid and early complete response to Polatuzumab and Rituximab. Bendamustine was withheld throughout treatment because of neutropenia except for a single dose during cycle 3. Complete metabolic response was obtained at end of treatment and was sustained for 5 months, allowing the patient to be referred for Chimeric Antigen Receptor -T cell therapy. The omission of Bendamustine has the potential to improve tolerability of the regimen and to reduce the likelihood of lymphocytopenia in patients being bridged to CAR-T cell therapy.
Keywords: Diffuse large B cell lymphoma; Relapsed; Polatuzumab; Bendamustine
Background
Relapsed and Refractory (R/R) Diffuse Large B Cell Lymphoma (DLBCL) has an extremely poor prognosis [1,2]. Treatment options include cisplatin based salvage chemotherapy in transplant eligible patients [3,4], and less intensive approaches including Bendamustine and Rituximab (BR) [5-8] for autologous transplant ineligible patients. Polatuzumab is an antibody drug conjugate targeting CD79b which, when combined with Bendamustine and Rituximab (Pola-BR) has been shown to have better clinical outcomes than BR alone in R/R DLBCL [9]. However, the role and contribution of Bendamustine in the Pola-BR regimen remains unclear. We present a patient with R/R DLBCL who had raid and sustained response to Polatuzumab and Rituximab (Pola-R) without significant side effects, providing anecdotal evidence that Bendamustine can be safely omitted without loss of efficacy. This could allow patients to be bridged to Chimeric Antigen Receptor T (CAR-T) cell therapy.
Case Report
A 75 year old lady was first diagnosed with Germinal Centre (GC) DLBCL in November 2015 when she presented with a large pelvic mass compressing the left ureter and causing acute renal failure. She had normal counts, no bone marrow involvement, stage 3 disease on computed tomography/ positron emission tomography, and Revised International Prognostic Index (R-IPI) of 3. She had a background history of mild rheumatoid arthritis, gastro-oesophageal reflux, and right leg deep venous thrombosis treated with Clexane.
She was treated with 6 cycles of Rituximab, Cyclophosphamide, Doxorubin, Vincristine, Prednisolone (R-CHOP) and ureteric stenting, with good initial response. She received Ifosfamide, Carboplatin and Etoposide (ICE) regimen [10] for first relapse in August 2016, and Filgrastim, Gemcitabine, Ifosfamide and Vinorelbine (FGIV) salvage chemotherapy [11] for refractory disease in November 2016 with short lived responses. Progressive disease with gradual increase in pelvic mass, bony invasion and worsening pain and chronic renal failure secondary to ureteric compression precluded eligibility for clinical trials. She received palliative radiotherapy in December 2017 with improvement in pain but otherwise negligible clinical benefit. In May 2018, she was commenced on single agent Selinexor [12] with which she had partial objective and subjective response. Treatment was complicated by severe treatment related cytopenias with platelets of ≺ 50 X 109/L, requiring weekly Romiplostim and platelet transfusions. Within 6 months of starting Selinexor, she was found to have asymptomatic progressive disease on surveillance PET scan performed in December 2018 (Figure 1A).
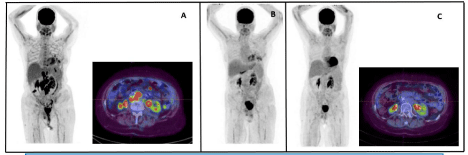
Figure 1:
So far, patient had received 5 lines of treatment and had refractory disease. Over the next few weeks, her Eastern Cooperative Oncology Group (ECOG) performance status worsened to 2 and she required pain medications including gabapentin, hydromorphone and prednisolone, and a walker for mobilisation.
In January 2019, she was commenced on two 21-day cycles of Pola-Rituximab at standard doses [9] with omission of Bendamustine because of persistent cytopenias. The first cycle was complicated by transient worsening of renal function unaccompanied by other biochemical markers of tumour lysis, and by an episode of febrile neutropenia which was treated with IV antibiotics.
She went on to receive 4 additional cycles of Pola-R at 21 day intervals with 2 brief episodes of uncomplicated febrile neutropenia and urosepsis, which responded to intravenous IV and oral antibiotics.
Clinically, the patient had a remarkable response to Pola-R with marked reduction in pain, improvement in general sense of wellbeing, and improvement in renal function and blood counts. PET scan performed after cycle 2 on 8th March 2019 confirmed near Complete Metabolic Response (CMR) (Figure 1B).
The post treatment PET scan on 8th July 2019 demonstrated ongoing CMR (Figure 1C). She remained in remission for 5 months which allowed her crucial time for referral for CAR-T cell therapy.
If Polatuzumab based regimens are being used to bridge to CAR-T cell therapy, consideration should be given to avoiding Bendamustine and consequent T cell lymphocytopenia.
Discussion and Conclusion
Polatuzumab has a unique mechanism of action and in combination with Bendamustine and Rituximab, has been shown to improve median Progression Free Survival (PFS) to 9.5 mo (95% CI 6, 17) [BR = 2.0 mo (95% CI 1.5, 3.7), stratified HR 0.34 (95% CI 0.2, 0.57, p≺0.0001)] and median Overall Survival (OS) to 12.4 mo (95% CI 9.0, not estimable) [BR = 4.7 mo ( 5% CI 3.7, 8.3), stratified HR 0.42 (95% CI 0.24, 0.75, p-value 0.0023)] [13]. Two separate statistical methods to model clinical outcomes have estimated mean OS between 1.9 yrs (parametric model) and 4.7 yrs (mixture model) for Pola-BR, as opposed to 0.8 yrs (both methods) for BR, and estimated median OS beyond 2 years of 38% for the Pola-BR arm vs. 9% for the BR arm [14].
The depth and durability of response with Pola-BR is being monitored. At a median follow-up time of 22.3 months for the R/R DLBCL cohort, Pola-BR showed significantly higher PET-CR rates compared to BR (40% vs. 18%; p=0.026) at end of treatment with 6 patients (15%) having ongoing response durations of ≤20 months without additional treatment. The median duration of response as assessed centrally was 12.6 months (95% CI, 7.2 months, not estimable) [13].
We present a case of CMR occurring very early in the course of the treatment i.e. after 2 cycles of Pola-R, which suggests that Bendamustine is not vital to the regimen. This has been previously demonstrated in the ROMULUS study which showed that 8 of the 39 R/R DLBCL (21%, 95% CI 9–36) patients treated with Pola-R achieved CR [15]. While acknowledging that the results are from separate studies, the median duration of response in the ROMULUS study for Pola-R was somewhat similar to the phase 2 study at 13·4 months (95% CI 6·5–21·2).
This patients’ characteristics can be considered worse than that of the patient cohort in the phase 2 study [9]. She was 74 years of age, had ECOG performance status of 2, had stage 3 bulky disease and had received 5 previous lines of treatment for chemo-refractory DLBCL. As reported in the study at incidence rates of 40%, 12% and 19% respectively, our patient developed neutropenia, febrile neutropenia and infections [9].
While only `50% of patients completed the expected 6 cycles of treatment on study [9,14], our patient was able to complete all 6 cycles which may have been partly related to the omission of Bendamustine.
Bendamustine is a nitrogen mustard which is widely used in indolent lymphomas with good benefit [16,17]. It has been used less frequently in transplant ineligible R/R DLBCL [8,18]. Side effects of the drug include rash, fever and Steven Johnson like syndrome, bone marrow suppression and stem cell toxicity often causing prolonged cytopenias [8,18,19]. In particular, marked suppression of T cell counts has been reported to persist for up to 3 years, which predisposes patients to secondary infections [20]. Being able to omit Bendamustine has the added advantage of reducing the risk of infections.
Moreover, it is widely acceptable that this regimen is unlikely to be curative, and in patients suitable for transplant or for CAR-T cell therapy, it is probably best used as bridging therapy. As Bendamustine causes prolonged suppression of T lymphocytes, this can potentially impact on collection of T lymphocytes for CAR-T cell therapy. This may provide further argument for dropping Bendamustine from the treatment regimen, at least in a proportion of patients i.e. those who are at a high risk for neutropenia and/or infections, and those that are being bridged to CAR-T cell therapy. This case provides anecdotal reassurance that CMR can be achieved without the use of Bendamustine. Further data needs to be collected on clinical outcomes through clinical trials on patients who were unable to tolerate Bendamustine, and through real-world data.
Authorship Contributions
DT treated the patient, sought consent and written the first draft of the case report with AG providing radiological review and contributing to the paper.
References
- Larouche JF. Lymphoma recurrence 5 years or later following diffuse large B-cell lymphoma: clinical characteristics and outcome. J Clin Oncol. 2010; 28: 2094-2100.
- Coiffier B. Characteristics of Refractory and Relapsing Patients with Diffuse Large B-Cell Lymphoma. Blood. 2008; 112: 2589-2589.
- Gisselbrecht C. Salvage Regimens with Autologous Transplantation for Relapsed Large B-Cell Lymphoma in the Rituximab Era. Journal of Clinical Oncology. 2010; 28: 4184-4190.
- Crump M. Randomized Comparison of Gemcitabine, Dexamethasone, and Cisplatin Versus Dexamethasone, Cytarabine, and Cisplatin Chemotherapy Before Autologous Stem-Cell Transplantation for Relapsed and Refractory Aggressive Lymphomas: NCIC-CTG LY.12. Journal of Clinical Oncology. 2014; 32: 3490-3496.
- Crump M. Gemcitabine, dexamethasone, and cisplatin in patients with recurrent or refractory aggressive histology B-cell non-Hodgkin lymphoma. Cancer. 2004; 101: 1835-1842.
- Corazzelli G. Long-term results of gemcitabine plus oxaliplatin with and without rituximab as salvage treatment for transplant-ineligible patients with refractory/relapsing B-cell lymphoma. Cancer Chemotherapy and Pharmacology. 2009; 64: 907-916.
- Mounier N. Rituximab plus gemcitabine and oxaliplatin in patients with refractory/relapsed diffuse large B-cell lymphoma who are not candidates for high-dose therapy. A phase II Lymphoma Study Association trial. Haematologica. 2013; 98: 1726-1731.
- Ohmachi K. Multicenter Phase II Study of Bendamustine Plus Rituximab in Patients With Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Journal of Clinical Oncology, 2013. 31: 2103-2109.
- Sehn LH. Addition of Polatuzumab Vedotin to Bendamustine and Rituximab (BR) Improves Outcomes in Transplant-Ineligible Patients with Relapsed/ Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL) Versus BR Alone: Results from a Randomized Phase 2 Study. Blood. 2017; 130: 2821-2821.
- Kusano Y. ICE (Ifosfamide, carboplatin, and etoposide) was the Best Salvage Regimen in Patients with Relapsed or Refractory Malignant Lymphoma. Blood. 2014; 124: 5432-5432.
- Pasricha SR. A multicenter phase 2 study of risk-adjusted salvage chemotherapy incorporating vinorelbine and gemcitabine for relapsed and refractory lymphoma. Cancer. 2008; 113: 3192-3198.
- Maerevoet M. Single Agent Oral Selinexor Demonstrates Deep and Durable Responses in Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL) in Both GCB and Non-GCB Subtypes: The Phase 2b Sadal Study. Blood. 2018; 132: 1677-1677.
- Sehn LH. Polatuzumab Vedotin (Pola) Plus Bendamustine (B) with Rituximab (R) or Obinutuzumab (G) in Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Updated Results of a Phase (Ph) Ib/II Study. Blood. 2018; 132: 1683-1683.
- Sehn LH. Estimation of long-term survival with polatuzumab vedotin plus bendamustine and rituximab for patients with relapsed/refractory diffuse large b-cell lymphoma (r/r dlbcl). Hematological Oncology. 2019; 37: 257-258.
- Morschhauser F. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019; 6: e254-e265.
- Rummel MJ. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013; 381: 1203-1210.
- Rummel MJ, Richard Greil CL, Gºrner M. Bendamustine-Rituximab Induction Followed by Observation or Rituximab Maintenance for Newly Diagnosed Patients with Waldenstrºm’s Macroglobulinemia: Results From a Prospective, Randomized, Multicenter Study (StiL NHL 7-2008 –MAINTAIN-; ClinicalTrials.gov Identifier: NCT00877214). IWWM-8 Session 10, August 16, 2014, 2014.
- Vacirca JL. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol. 2014; 93: 403-409.
- Weidmann E. Phase II study of bendamustine in combination with rituximab as first-line treatment in patients 80 years or older with aggressive B-cell lymphomas. Ann Oncol. 2011; 22: 1839-1844.
- Martinez-Calle N. Kinetics of T-cell subset reconstitution following treatment with bendamustine and rituximab for low-grade lymphoproliferative disease: a population-based analysis. Br J Haematol. 2019; 184: 957-968.