
Case Report
Ann Hematol Oncol. 2020; 7(3): 1289.
5-HT3 Antagonist Related Erections and Myocardial Ischemia (HAREM Syndrome): A Tale of Caution of Palonosetron-Induced Priapism and Myocardial Infarction in a Patient with Acute Myeloid Leukemia
Yogakanthi S1, Tan J1*, William J2, Corallo3 and Wei A1
¹Department of Clinical Hematology, The Alfred Hospital, Melbourne, Australia
²Department of Cardiology, The Alfred Hospital, Melbourne, Australia
³Department of Pharmacy, The Alfred Hospital, Melbourne, Australia
*Corresponding author: Joanne Tan, Department of Clinical Hematology, The Alfred Hospital, Melbourne, Victoria, Australia
Received: February 10, 2020; Accepted: March 09, 2020; Published: March 16, 2020
Abstract
Palonosetron is a second-generation 5-HT3 antagonist that is commonly used in chemotherapy supportive care. Unlike other older 5-HT3 antagonists (e.g. ondansetron), the short duration of clinical experience means its adverse effect profile is less well established. Our case report describes the first case of palonosetron associated myocardial ischemia and priapism in a patient receiving induction chemotherapy. This case report highlights the cardiac and vascular adverse effects associated with 5-HT3 antagonists and the need for prompt recognition to avoid further administration of the agent and minimise harm.
Keywords: Anti-emetics; Troponin; Chest pain; Adverse drug reaction; Chemotherapy
Abbreviations
CINV: Chemotherapy Induced Nausea and Vomiting; 5-HT: 5-Hydroxytryptamine; ECG: Electrocardiogram; TTE: Transthoracic Echocardiogram; CTPA: Computed Tomography Pulmonary Angiogram; LV: Left Ventricle; RV: Right Ventricle; PCR: Polymerase Chain Reaction
Case Presentation
Palonosetron is a second generation 5-HT3 selective receptor antagonist with a significantly longer half-life (approximately 40 hours) and higher target binding affinity (up to 30 times higher) than first generation agents such as ondansetron [1]. Its extended efficacy in controlling delayed chemotherapy-induced nausea has prompted widespread incorporation into cytotoxic regimens for Acute Myeloid Leukemia (AML) [2]. Increased use of palonosetron, however, obliges medical practitioners to recognize rare but serious adverse effects, to manage these events appropriately and avoid repeated drug administration. Here we report a case of palonosetronassociated myocardial infarction in a patient undergoing induction chemotherapy for AML.
A 57-year-old male was diagnosed with AML in the context of incidental pancytopenia. They reported a good premorbid exercise tolerance with no cardiac risk factors and no personal or family history of cardiac disease. Radionuclide ventriculography performed one day prior to chemotherapy commencement showed a normal Left Ventricular (LV) ejection fraction of 74% and no regional wall motion abnormalities. A baseline Electrocardiogram (ECG) was unremarkable. The patient was commenced on 7+3 intensive induction (cytarabine 200mg/m2 continuous intravenous [IV] infusion D1-7 and idarubicin 12mg/m2 IV D1-3) as per protocol. A combination oral antiemetic drug, netupitant (300mg)/palonosetron (0.5mg), was administered prophylactically on day 1. Concomitant medications commenced prior to induction included allopurinol, pantoprazole and valaciclovir while posaconazole was commenced on day 3.
The evening after receiving palonosetron, the patient experienced episodic intractable priapism that persisted for approximately 4 hours on two consecutive nights. Episodes of recurrent nonexertional central chest tightness lasting an hour developed 24 hours post priapism onset, persisting over the subsequent three days before resolving. Serial troponins and an ECG performed at pain onset showed no evidence of cardiac ischemia. A further dose of IV palonosetron (0.25 mg) was administered on day 4. Three hours after palonosetron, a troponin level performed in the setting of chest tightness was elevated at 370ng/L (upper limit of normal 26 ng/L), with sinus tachycardia (101 beats per minute) and new T wave inversion in Lead III. A CT pulmonary angiogram showed no evidence of pulmonary embolus. The ECG changes evolved over the subsequent 12 hours to ST elevation in Leads II, aVF, V3-V4 along with T wave inversion in aVL. A Transthoracic Echocardiogram (TTE) performed on day 5 demonstrated bi-ventricular dysfunction with low to normal LV and moderately reduced Right Ventricular (RV) systolic function. Emergent coronary angiography showed no evidence of obstructive coronary artery disease.
Subsequent ECGs demonstrated resolution of ischaemic changes with a troponin peak of 25,817ng/L on day 5, 24 hours post the second dose of palonosetron (Figure 1). A follow-up TTE on day 9 showed interval normalisation of global LV and improvement from moderate to mild RV dysfunction. LV global longitudinal strain was low-normal at -16.5% with segmental strain abnormalities affecting the anteroseptum and inferoseptum (Figure 2). Peripheral blood PCR for coxsackievirus, adenovirus and cytomegalovirus were negative. Following full blood count recovery post induction high dose cytarabine consolidation (3g/m2 BD D1, 3, 5) was administered without 5-HT3 antagonists. No further chest pain episodes occurred.
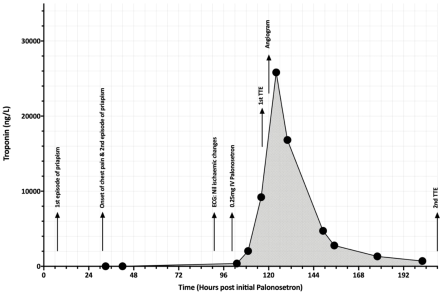
Figure 1: Troponin trend in relation to timeline of key clinical events and palonosetron administration.
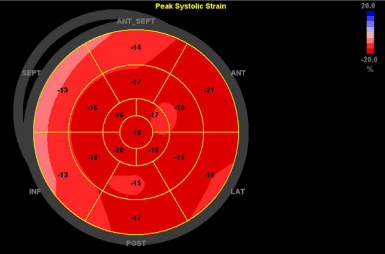
Figure 2: Bull’s eye diagram of global longitudinal strain illustrating levels
of ventricular myocardium shortening. Increasingly positive numbers (and
lighter shades of red) reflect diminishing longitudinal shortening. Hypokinesis
of the septum is shown in the left upper quadrant of the image (pink).
Discussion
Our case is a cautionary tale linking 5-HT3 antagonists to priapism and myocardial ischemia, consistent with the rare but known cardiotoxicity of 5-HT3 antagonists. Previous reports have detailed adverse events including cardiac arrythmias and myocardial infarction [3-5]. Definitive causality is difficult to ascertain due to concomitant medication administration and comorbidities. The predominant agent implicated in these reports has been IV ondansetron, although a randomised controlled trial investigating granisetron in children demonstrated transient asymptomatic ECG changes, suggesting a broader class effect [6].
An assessment of co-administered medications in our patient did not identify an alternative candidate drug, with piperacillintazobactam and oxycodone being the other newly administered agents on the day of the event. The Naranjo score was 7, suggesting a probable likelihood secondary to palonosetron [7]. Stress (Takotsubo) cardiomyopathy, characterised by chest pain and regional LV systolic dysfunction in the absence of coronary artery disease, occurs more frequently in patients receiving chemotherapy [8]. However, the pattern of segmental systolic dysfunction in our case (restricted to the septum with apical sparing) is atypical. Idarubicin-induced cardiomyopathy was another differential, however the regional ECG changes and wall hypokinesis were more consistent with singlevessel ischemia than cardiomyopathy. Acute viral myocarditis is more frequent in patients with haematological malignancies [9]. However, the pattern of regional LV systolic dysfunction, normal LV wall thickness, negative viral PCR screens and rapid recovery of ventricular function made this unlikely.
The mechanisms leading to ischemia through use of 5-HT3 antagonists are not clearly defined with evidence predominantly from animal studies [10]. The major cardiac effect of 5-HT is a bradycardiainducing Bezold-Jarisch-like reflex mediated by 5-HT3 receptors on cardiac vagal afferents. 5-HT3 antagonist-related ischemia is postulated to occur via inhibition of this reflex, which leads to tachyarrhythmias. When combined with coronary vasoconstriction mediated by the un-antagonised additional 5-HT receptor subtypes, the likelihood of cardiac ischemia is increased. [11]. This myocardial oxygen demand-supply mismatch explains the ischaemic prodrome and subsequent infarction in our patient despite the lack of coronary artery disease. Fortunately, his cardiac dysfunction was reversible and improved to near normal at 5 days post drug exposure. Priapism related to 5-HT3 antagonists has only been described in a few cases, all of whom received IV ondansetron. Possible mechanisms involve inhibition of cavernosal smooth muscle relaxation and antagonism of central serotoninergic pathways involved in erectile function [12,13]. All reported cases had spontaneous symptom resolution following 5-HT3 antagonist therapy cessation, as observed in our patient.
Conclusion
This case illustrates the need for physician awareness of palonosetron-related vascular side-effects and the need to prevent repeated drug exposure in subsequent cycles of emetogenic chemotherapy. The unusual co-occurrence of priapism and cardiac ischemia should alert to the possibility of a medication-related systemic vasoactive aetiology, rather than a localised coronary occlusive episode. The syndromic recognition of 5-HT3 Antagonist Related Erections and Myocardial ischemia (HAREM syndrome) temporally related to anti-emetic drug administration should trigger immediate consideration of alternative prophylactic antiemetic options in patients receiving chemotherapy.
Acknowlegements/Author Contributions
Saiumaeswar Yogakanthi participated in writing of the manuscript
Joanne Tan participated in writing of the manuscript
Jeremy William participated in writing of the manuscript
Carmela Corallo participated in writing of the manuscript
Andrew Wei participated in revising the manuscript
References
- Musso M, Scalone R, Crescimanno A, Bonanno V, Polizzi V, Porretto F, et al. Palonosetron and dexamethasone for prevention of nausea and vomiting in patients receiving high-dose chemotherapy with auto-SCT. Bone Marrow Transplant. 2010; 45: 123-127.
- Mattiuzzi GN, Cortes JE, Blamble DA, Bekele BN, Xiao L, Cabanillas M, et al. Daily palonosetron is superior to ondansetron in the prevention of delayed chemotherapy-induced nausea and vomiting in patients with acute myelogenous leukemia. Cancer. 2010; 116: 5659-5666.
- Baguley WA, Hay WT, Mackie KP, Cheney FW, Cullen BF. Cardiac dysrhythmias associated with the intravenous administration of ondansetron and metoclopramide. Anesth Analg. 1997; 84: 1380-1381.
- Guigui N, Luyt CE, Vincent F. Coronary spasm after injection of ondansetron: case report and review of the literature. Int J Cardiol. 2008; 123: 341-342.
- Palmer JB, Greenstreet YL. Ondansetron and chest pain. Lancet. 1992; 340: 1410.
- Buyukavci M, Olgun H, Ceviz N. The effects of ondansetron and granisetron on electrocardiography in children receiving chemotherapy for acute leukemia. Am J Clin Oncol. 2005; 28: 201-204.
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30: 239-245.
- Desai R, Abbas SA, Goyal H, Durairaj A, Fong HK, Hung O, et al. Frequency of Takotsubo Cardiomyopathy in Adult Patients Receiving Chemotherapy (from a 5-Year Nationwide Inpatient Study). Am J Cardiol. 2019; 123: 667- 673.
- Marchesi F, Pimpinelli F, Ensoli F, Mengarelli A. Cytomegalovirus infection in hematologic malignancy settings other than the allogeneic transplant. Hematol Oncol. 2018; 36: 381-391.
- Havrilla PL, Kane-Gill SL, Verrico MM, Seybert AL, Reis SE. Coronary vasospasm and atrial fibrillation associated with ondansetron therapy. Ann Pharmacother. 2009; 43: 532-536.
- Saxena PR, Villalon CM. 5-Hydroxytryptamine: a chameleon in the heart. Trends Pharmacol Sci. 1991; 12: 223-227.
- Kilciler G, Kilciler M, Kadihasanoglu M, Atahan O. Nonischaemic priapism associated with selective serotonin-3 receptor antagonist. Andrologia. 2017; 49.
- Pivot D, Javot L, Swiegot D, Petitpain N, Spaeth D, Trechot P. Two cases of recurrent priapism during antineoplastic chemotherapy: think about ondansetron. Therapie. 2013; 68: 409-410.