
Research Article
Ann Hematol Oncol. 2020; 7(3): 1291.
Response to Front and Second Line Treatment in Patients with Acute Non Lymphoblastic Leukemia: A Single Center Experience
Elyamany A1, Khaled SA2*, Farghaly R1 and Abdallah AZ3
¹Department of Medical Oncology, South Egypt Cancer Institute, Assiut University, Egypt
²Department of Internal Medicine, Clinical Hematology Unit, Assiut University Hospital/BMT Unit, South Egypt Cancer Institute, Faculty of Medicine, Assiut University, Egypt
³Department of Clinical Oncology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt/Oncology consultant, KSMC-Riyadh-KSA
*Corresponding author: Safaa A.A. Khaled, Department of Internal Medicine, Clinical Hematology Unit, Assiut University Hospital, Faculty of Medicine, Assiut University, Assiut, Egypt
Received: February 22, 2020; Accepted: March 16, 2020; Published: March 23, 2020
Abstract
Background: Acute Non- Lymphoblastic (ANLL) is the type of leukemia with the worst prognosis. Over the past 30-years, treatment of ANLL consisted of two phases, frontline therapy aimed to achieve leukemic cell clearance, and second line to prevent relapse. This study assessed response to front and second line therapy in patients with ANLL.
Methods: Data of 90- ANLL patients were retrospectively collected from hospital records of those who were admitted at the Department of Medical Oncology, South Egypt Cancer Institute (SECI) in the period 2000-2010. Patients were treated with frontline induction regimens, and second line therapy in the form of HAM (33.9%), consolidation for M3 (6.5%). 59.7% of our patients discontinue treatment.
Results: 68.9% of patients achieved Complete Remission (CR) that was different among different induction courses. Patients who received HAM, 17 continue remission for a period from 3-18 month till BMT. Those didn’t received HAM, 11 patients suffered from relapse. The longest overall (OS) and Disease Free Survival (DFS) were 20 and 18 month in 10.6% and 1.1% respectively. Those who achieved CR after first 1st induction had longer OS and DFS, P <0.05.
Conclusion: This study concluded that early blast clearance is a good prognostic factor in ANLL. Also it encouraged post remission therapy with HAM to lengthen DFS till HSCT. However patient incompliance was a problem, accordingly efforts has to be exerted to keep patients in regular treatment.
Keywords: ANLL; Frontline therapy; Second line
Introduction
ANLL is a hematopoietic myeloid stem cell disorder in which the bone marrow is flooded wih immature myeloid lineage cells that interfer with the production of normal blood cells [1]. For the past 30 years, and uptill now, treatment of ANLL has generally consisted of two phases. The first phase attempts to produce CR and named the induction phase. CR is defined as a bone marrow blasts less than 5% blasts, a neutrophil count greater than 1000, and a platelet count greater than 100 000. CR is the only response that leads to cure and, at the least, to an extension in survival. This can be achieved in about 60–80% of patients with ANLL with intensive chemotherapy regimens, but at least 30% of these patients will develop relapse thereafter. The second phase of therapy aims to prolong the CR. Once a patient has been in remission for 3 years, the likelihood of relapse declines sharply to less than 10% [2].
SECI is a tertiary health center that provides cancer care and treatment to patients with various malignanacies at Upper Egypt. A recent study in Upper Egypt found that only 16.3% only of ANLL received consolidation therapy [3]. Another study at Kasr Al Ainy center of clinical oncology and nuclear medicine in the period 2010- 2014, showed that the CR of patients with ANLL was 65%, 12.5% early deaths, 13.8% with refractory disease and the OS was 11.5 months, furthermore half of remitent patients relapsed [4].
As far as we know, this is the first study that assesed response to front and second line treatment in adult patients with ANLL at SECI and adressed clinical challenges of patient managment at this big institute.
Patients and Methods
Data of ANLL patients who were admitted and treated at SECI from 2000 to 2010 were retrospectively collected from patients’ files, then arranged and recorded on a new sheet for the study. The extracted data included patients’ demographics and ECOG (Eastern Cooperative Oncology Group) performance status. Type of ANLL either by morphology, immunophenotyping and data concerned with drugs used in induction and consolidation were also collected. Particular attention was paid to bone marrow results to document the following: Diagnosis, CR, relapse, and PFS. Unfortunately, cytogenetic and molecular profiles were unavailable for most of the patients thus were omitted. Each record was thoroughly examined to collect the date of death and have the OS. Multiple records of the same patient, who was admitted more than once, were considered as a single record to obtain a full course of the disease.
PFS is the time from the start of treatment to the first documentation of objective tumor progression. OS is the time from start of treatment to date of death as a result of any cause or last follow up.
Statistical Analysis
The collected data were verified, coded then analyzed by using the Statistical Package for Social Sciences (SPSS/PC/VER 17) also a recent version of GraphPad Prism was used. Nevertheless, some records were incomplete others included missing data, accordingly they were excluded. Descriptive statistics: mean, standard deviation, frequencies, percentage were calculated. Kaplan-Mayer and Survival analysis was calculated. Significant test results were considered when P value was < 0.05.
Ethical Considerations
The study protocol and methods were consistent with the World Medical Association Declaration of Helsinki, also were approved by the Department of Higher affairs at SECI. Moreover, the study received approval from the Ethics Committee of the Faculty of Medicine, Assiut University. However, as the study was retrospective patient consent was inappropriate. Confidentiality was assured.
Results
Demographic and disease characteristics of the study patients
Table 1, showed demographic clinical and disease characteristics of 90 patients with ANLL at SECI from 2000– 2010. The mean age was (37.9±6.9 years), 51.1% were males. Residence ANLL patients at SECI from 2000 – 2010¸ 44 patients were from Assiut (48.9 %)¸ 19 from Sohag (21.1%)¸ 8 from Qena (8.9 %)¸ 5 from Aswan (5.6 %)¸ 4 from El-Menia (4.4%)¸ 1 patient from El-wady (1.1 %)¸ 1 patient from Red sea governorate (1.1 %), data on residence was missed in 8 patients (8.9 %). ECOG performance status was 1 in 76 patients (84.4 %) and 2 in 14 patients (15.6 %). TLC for the study patients was <100.000 in 86 patients (95.6%) and 4 patients had leukocytic count >100.000 (4.4%). Only 5 patients had CNS infiltration (5.6%).
TABLECREATED
Table 1: Demographic and disease characteristics of 90 patients with ANLL at SECI from 2000– 2010.
Figure 1 showed type and subtypes of ANLL, there was 2 patients with M0 (2.2%), 12 patients with M1 (13.3%), 19 patients with M2 by (21.1%),19 with M3 (21.1%), 16 patients with M4 (17.8%), 8 patients with M5 (8.9%), 5 patients with M6 (5.6%), 1 patient with M7 by (1.1%) and1 patient with CML with blastic transformation (1.1%) (Table 1).
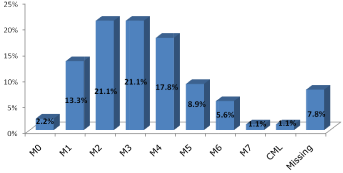
Figure 1: Type and FAB subtype of ANLL in the study patients.
Response of the study patients to frontline treatment
90 patients received induction, among them 50 (55.5%) patients showed remission after first induction and 40 patients showed no remission. Second induction was given to 16 patients who didn’t show remission after first induction, remission occurred in 10 (62%) patients. 2 patients received third induction both of them showed remission, as in (Figure 2). Accordingly response to frontline treatment of ANLL patients at SECI (2000-2010) was CR in 68.9%.
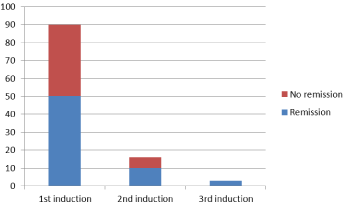
Figure 2: Response to different courses of frontline therapy in the study
patients.
Response to second line therapy in the study patients
Out of the 62 patients who showed remission 21 patients received HAM regimen (33.9%), among the remaining 4(6.5%) patients diagnosed as M3 and received different consolidation regimens, more than half of the patients (37(59.7%)) discontinue treatment, (Figure 3). Patients who received HAM, 17 patients continue remission for a period ranging from 3 to 18 month till bone marrow transplantation. While patients who didn’t received HAM, 11 patients suffered from relapse (Figure 3).
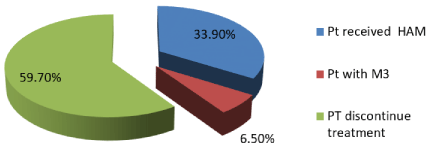
Figure 3: ANLL Patients at SECI (2000-2010) who received secondline
therapy.
Assessment of relapse in the study patients showed no relapse in 71(78.8%) patients and relapse in 19 (21.1%) patients. Outcome of ANLL patients, there was 49 patients alive (54.4%) and still under follow up (during the period of the study) and 41 patients died (45.5%).
Survival analysis of the study patients
Overall survival: In our study the maximum period of follow up was 20 months with survival proportion 10.6%, while half of the patients continue follow up for 10 months, (Table 2 and Figures 4&5).
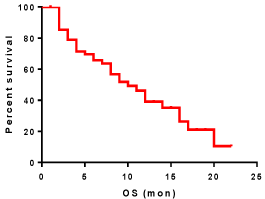
Figure 4: Overall survival of the study patients.
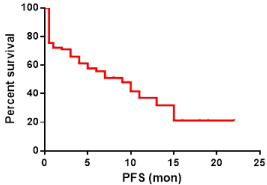
Figure 5: Overall survival according to courses of front line therapy in the
study patients.
TABLECREATED
Table 2: Overall survival of ANLL patients included in the study.
Progression free survival of anll: Median duration without progression of the patients under study is estimated to be 9 months. As the courses of treatment spent around 6 months (2 inductions and 3 consolidation) this means that half of patients suffer from relapse within 4 months. Only 1 patient remained without relapse for around one and half year, (Table 3, Figure 7).
TABLECREATED
Table 3: Progression free survival of the study patients.
Survival analysis according to courses of front line therapy in the studied sample: There was no statistically significant difference between courses of front line therapy in OS however patients who showed remission after first induction had a trend for longer survival duration (median survival =16 month), (Table 4 and Figure 6).
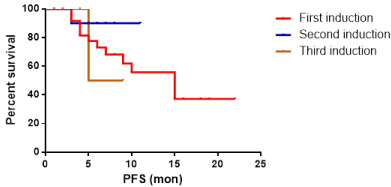
Figure 6: Progression free survival according to courses of front line therapy
in the study patients.
TABLECREATED
Table 4: Overall survival according to courses of front line therapy in the study patients.
As described above similar trend was seen in progression free survival with median survival after first induction was 15 months, P value =0.5, (Table 5 and Figure 7).
TABLECREATED
Table 5: Progression free survival according to courses of front line therapy in the study patients.
Discussion
ANLL is the most common type of leukemia in adults, yet continues to have the lowest survival rate of all leukemia’s [5]. Regarding the Arab world, Statistics have shown that leukemia is one of the 10 most common malignancies in Egypt and many other Arabic countries [6]. It is estimated that there are around 3,335 new cases per year in Egypt [7]. The conventional treatment of ANLL, in many centers worldwide, consisted of front line and second line therapy [8]. In our study we tried to assess the response to these therapies in ANNL cases treated at SECE and their implication on patient survival. Accordingly, we retrospectively collected data of 90- patients with ANLL who were admitted at SECI in the period 2000- 2010.
Analysis of the collected data showed male predominance percentage, ANLL in adults has a slight male predominance in most countries. The incidence rate of U.S. males is substantially higher than the incidence rates reported for males in all other countries. The exact significance of this gender preference is not clear [9,10].
In our study, the most common FAB subtypes were M2 (21.1%) and M4 (17.8%). This is the same reported with FAB classification in USA and UK, [11] but M3(21.1%) is also high as SECI is the tertiary referral center at Upper Egypt which has the facilities to treat this type but the other types can be managed in other centers.
ANLL influences all ages but is mainly a disease of the elderly with a median age of 69 years in the white US population, but in our study the median age was 37 years. This median age was albeit consistent with Khaled et al., [3]. The life expectancy of Egyptians is 72 years compared to 78 years for the Americans and almost 95% of the Egyptians are below 60 years compared to 13% for the Americans [12]. So usually at SECI the number of young patients is more than the old patients, and usually young adults seek medical advice more than the elderly. Furthermore, younger adults may be exposed to hazardous chemicals (benzene) or to radiation [13].
Patients who show remission after first induction course survive for a longer duration without relapse than those showing remission for the first time after the second or third induction. Early response to chemotherapy has a major prognostic impact in patients with ANLL. Failure to achieve early blast clearance remains a poor prognostic factor even after early salvage [14].
More recently, long-term results of the multicenter prospective LAM-2001 trial by the GOELAMS study group showed that Patients with fewer than 5% blasts measured on the 15th day after treatment (d15-blasts) had a higher complete response rate (91.7% vs. 69.2%; P<0.0001) and a lower induction death rate (1.8% vs. 6.8%; P=0.001). Five-year event-free (48.4% vs. 25%; P<0.0001), relapse-free (52.7% vs. 36.9%; P=0.0016) and overall survival (55.3% vs. 36.5%; P<0.0001) were significantly higher in patients with d15-blasts lower than 5%. Multivariate analyses identified d15-blasts and cytogenetic risk as independent prognostic factors for the three end points [15].
In the current study the CR of ANLL patients was 68% which was consistent with the reported CR in many studies that ranged from 65- 70%, 25% of those could survive more than 3-years [16-18].
Many studies concluded the importance of cytogenetic and molecular analyses in treatment decision for ANLL patients [16,19- 22], however this was not available for all patients at the time of this study. According to treatment policy in SECI ANLL patients who show remission after induction are planned to receive consolidation regimen in the form of High Dose Cytarabine (HAM). Only one third of patients who showed remission after induction received HAM. Due to false sensation of complete cure and lack of desire to receive more chemotherapy many of our patients discontinue treatment. Patients who received HAM can survive for a longer duration till bone marrow transplantation can be done for them as long as 18 months.
According to cooperative group MRC16, SWOG/ECOG19 and CALGB23, risk stratification of karyotype abnormalities in patients of ANLL after first remission (CR1), is used to determine post remission treatment. Treatment of choice for unfavorable risk group is allogeneic HSCT [19]. In such case there is no need for further consolidation chemotherapy which is lengthy, toxic and not effective in this group of patients. Applying this risk stratification policy in our management protocol for ANLL may be of benefit for a group of patients who discontinue or cannot withstand further chemotherapy [23,24].
In Egypt facilities that can provide bone marrow transplantation is very limited. In general, ANLL patients should wait from 6 to 12 month or even more for transplantation. Therefore, consolidation treatment is required for such patients to provide relapse free duration as long as possible till they can do bone marrow transplantation, provided good selection of candidate as described above.
Conclusion
In conclusion this study showed that early remission after first course of front line therapy carried better prognosis in ANLL patients who show remission after second and third course. Moreover, it revealed that consolidation treatment provided a longer duration without relapse, while waiting for bone marrow transplantation. Nevertheless, cytogenetic profile and risk stratification are crucial for directing post remission management of ANNL.
References
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006; 368: 1894-907.
- Kern W, Voskova D, Schoch C, Hiddemann W, Schnittger S, Haferlach T. Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood. 2004; 104: 3078- 3085.
- Khaled SA, Nabih O, Abdel Aziz NM, Mahran DG. Myeloid leukemias: a glance at Middle Eastern Centers. J Blood Med. 2019; 10: 425-433.
- Zawam H, Salama R, Al Sirafy SA, Bishr MK. Treatment of acute myeloid leukemia in Egypt: A developing country perspective. Int J Cancer Treat. 2018; 1: 33-59.
- Redaelli A, Lee JM, Stephens JM, Pashos CL. Epidemiology and clinical burden of acute myeloid leukemia. Expert Rev Anticancer Ther. 2003; 3: 695- 710.
- Tadmouri GO. Cancers in Arab Populations: Concise Notes. Hamdan Medical Journal. 2012; 5: 79–82.
- Herzog C, Dey S, Hablas A, Khaled H, Seifeldin I, Ramadan M, et al. Geographic distribution of hematopoietic cancers in the Nile delta of Egypt. Annals of oncology. 2012; 23: 2748-2755.
- Rao AV, Valk PJ, Metzeler KH. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009; 27: 5580–5586.
- Parkin DM, Ferlay J, Whelan SL, Storm HH. Cancer Incidence in Five Continents, World Health Organization. 2005.
- Dohner H, Estey EH, Amadori S. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European Leukemia Net. Blood. 2010; 115: 453-474.
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia A report of the French-American-British Cooperative Group. Ann Intern Med. 1985; 103: 620-625.
- CAPMAS. Population Clock [Online]. Central Agency for Public Mobilization and Statistics CAPMAS. 2014.
- Tadmouri. Cancers in arab populations: concise notes. Hamdan medical journal. 2012; 5: 79–82.
- Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003; 101: 64-70.
- Bertoli S, Bories P, Bene MC, Daliphard S, Lioure B, Pigneux A, et al. Prognostic impact of day 15 blast clearance in risk-adapted remission induction chemotherapy for younger patients with acute myeloid leukemia: long-term results of the multicenter prospective LAM-2001 trial by the GOELAMS study group. Haematologica. 2014; 99: 46-53.
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood. 1998; 92: 2322-2333.
- Desantis CE, Lin CC, Moriotto AB. Cancer treatment and surviorship statistics. CA: a cancer journal for clinicians. 2014; 64: 252-271.
- Elzawahry HM, Zeen eldin AA, Samra MA. Cost and outcome of treatment of adults with acute myeloid leukemia at the National Cancer Institute Egypt. J Egypt Nat Canc Inst. 2009; 19: 106-113.
- Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/ Eastern Cooperative Oncology Group Study. Blood. 2000; 96: 4075-4083.
- Khaled SA, Burthem J, Abo ElNoor E. Differences between nucleophosmin isoforms in de-novo acute myeloid leukemia: possible implications in developing targeted therapy for acute myeloid leukemia with normal karyotype. Egyptian J Hematol. 2015; 40: 190-194.
- Kumar CC. Genetic abnormalities and challenges in the treatment of acute myeloid leukemia. Genes Cancer. 2011; 2: 95-107.
- Khaled SA, Burthem J, Abo ElNoor E. Role of nucleophosmin gene mutation in leukemogenesis of acute myeloid leukemia. J Hematol. 2018; 7: 7-13.
- Marcucci G, Mrozek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leukemia Group B study. J Clin Oncol. 2005; 23: 5705-5717.
- Basara N, Schulze A, Wedding U, Mohren M, Gerhardt A, Junghanss C, et al. Early related or unrelated haematopoietic cell transplantation results in higher overall survival and leukaemia-free survival compared with conventional chemotherapy in high-risk acute myeloid leukaemia patients in first complete remission. Leukemia. 2009; 23: 635-640.