
Rapid Communication
Ann Hematol Oncol. 2020; 7(4): 1296.
Clinical Impact of NPM1 Mutation Subtypes in a Monocentric Cohort of Acute Myeloid Leukemia
Sciumè M1*, Fabris S1, Ciceri G1, Mattiello V1,2, De Roberto P1, Neri A1,2, Baldini L1,2 and Fracchiolla NS1
¹Hematology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy
²Università Degli Studi di Milano, Italy
*Corresponding author: Sciumè M, Hematology Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Via Francesco Sforza 35, 20122 Milano, Italy
Received: April 22, 2020; Accepted: May 20, 2020; Published: May 27, 2020
Abstract
Acute Myeloid Leukemia (AML) with mutated Nucleophosmin Member 1 (NPM1) was recently recognized as a distinct entity. The most common NPM1 mutation is type A, generally considered of favorable prognosis. We aimed to evaluate the relevance of A and non-A type NPM1 mutations on clinical profiles and outcomes in a monocentric cohort of adult patients with newly diagnosed NPM1-mutated AML. We identified 30 consecutive patients over a 4-year period. Type A mutations were found in 22 cases and non-A type in 8 cases. Thirteen patients received intensive chemotherapy (8 type A, 5 non-A type) with Complete Remission (CR) in 10 cases (5 type A, 5 non-A type), while 11 patients received a hypomethylating agent (9 type A, 2 non-A type) with CR in 2 type A cases. Median Overall Survival (OS) and disease free survivals were 6.5 and 9 months; OS showed a trend towards a statistical significance for non-A type cases. Despite the limited number of patients, our study suggests that NPM1 genotypes had no impact in terms of CR rates after intensive chemotherapy; the possible association of NPM1 mutation type with OS deserves further investigations on larger series.
Keywords: Acute myeloid leukemia; NPM1 mutation; Prognosis
Abbreviations
NPM1: Nucleophosmin Member 1; AML: Acute Myeloid Leukemia; ITD: Internal Tandem Duplication; FLT3: Fms-Related Tyrosine 3 Gene; NES: Nuclear Export Signal; MRD: Minimal Residual Disease; OS: Overall Survival; DFS: Disease Free Survival; WBC: White Blood Cell Count; CR: Complete Remission
Introduction
The nucleophosmin Member 1 (NPM1) is a nucleolar protein with pleiotropic functions in ribosome biogenesis, messenger ribonucleic acid processing, chromatin remodeling, apoptosis and genome stability [1-3]. Acute Myeloid Leukemia (AML) with mutated NPM1 was recognized as a distinct entity by the 2016 revision to the World Health Organization classification of acute leukemia [4]. It accounts for 50-60% of de novo normal-karyotype AML and it is generally associated to a favorable prognostic impact when additional Internal Tandem Duplication (ITD) in the Fms-Related Tyrosine 3 gene (FLT3) gene is absent [4-6]. Almost all NPM1 mutations occur within exon 12 and involve frameshift insertions; these mutations cause a change in the NPM1 protein sequence and an early truncation in the Nuclear Export Signal (NES) motif, resulting in a cytoplasmic localization of the protein [1-3,5]. Approximately 80% of NPM1 mutations are type A (frameshift mutation due to tandem duplication of a TCTG tetranucleotide sequence), followed by 10% of type B (CATG) or type D (CGTG). Other rare mutations can be found in the remaining 10% [1-3,5]. So far only few studies investigated the prognostic role of different subtype of NPM1 mutations, with controversial results [7-9].
Materials and Methods
The aim of the present study was to assess the association of A type versus non-A type NPM1 mutations with specific clinical features and their relevance on outcomes in a monocentric cohort of AML consecutive patients. NPM1 mutations were detected by Sanger sequencing and molecular Minimal Residual Disease (MRD) was determined by real-time quantitative polymerase chain reaction analysis (sensitivity 10-5). The 2017 European Leukemia Net guidelines was used to assess the genetic risk classification and response to treatment [10]. Relationships between categorical variables were assessed by chi-squared test; p values <0.05 were considered significant. Overall Survival (OS) and Disease Free Survival (DFS) curves were estimated using the Kaplan–Meier method. the study was approved by ethic committees of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico Milano (711_2018). The research was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2013.
Results and Discussion
Thirty NPM1-mutated AML patients consecutively diagnosed at our Hematology Department between July 2014 and April 2019 were identified in the institutional database. Clinical data, diagnostic workup and treatment modalities are shown in (Table 1).
Characteristics
Total cohort
(30 patients)
Type A
(22 patients)
Type non-A
(8 patients)
Male/Female
15/15
13/9
6/2
Age (years), median (range)
70 (34-86)
73 (42-86)
63 (34-81)
White blood cell count (x109/L), median (range)
29 (0.8-444)
33 (0.8-444)
29 (1.6-155)
Bone marrow blasts (%), median (range)
60 (20-90)
60 (20-85)
80 (38-90)
French–American–British classification, n (%)
M1
M2
M4
M5
M6
6 (20)
4 (13)
16 (54)
3 (10)
1 (3)
5 (23)
1 (4)
13 (59)
3 (14)
0
1 (13)
3 (37)
3 (37)
0
1 (13)
Cytogenetics, n (%)
Normal
Intermediate-risk abnormalities
Failure
25 (84)
4 (13)
1 (3)
19 (87)
2 (9)
1 (4)
6 (75)
2 (25)
0
Type of NPM1 mutation †, n (%)
A (c.860_863dupTCTG)
B (c.863_864insCATG)
D (c.863_864insCCTG)
c.863_864insTTTG
c.863_864insTGCG c.870_873delGAGGinsCTTCTCCC ‡
22 (74)
3 (10)
2 (7)
1 (3)
1 (3)
1 (3)
FLT3-ITD mutation, n (%)
9 (30)
7 (32)
2 (25)
Type of treatment, n (%)
Intensive chemotherapy
Hypomethylating agents
Best supportive care
13 (43)
11 (37)
6 (20)
8 (36)
9 (41)
5 (23)
5 (62)
2 (25)
1 (13)
Table 1: Clinical and biological characteristics of A type and non-A type NPM1-mutated patients.
Median age at AML diagnosis was 70 years and patients were equally distributed by gender (male/female 15/15). Median White Blood Cell Count (WBC) was 29x109/L. Twenty-two (73%) patients showed a type A NPM1 mutations, while non-A type occurred in 8 (27%) cases, in particular B type (3 cases), D type (2 cases) and other rare mutations (3 cases). One of the non-A type mutation was novel: c.870_873delGAGGinsCTTCTCCC. The putative novel mutant protein was associated with a variant of the most common NES motif (i.e., L-xxx-V-xx-V-x-L) and showed a tryptophan loss at codon 290 only, resulting in aberrant cytoplasmic localization as confirmed by immunohistochemical analysis (data not shown).
Hyperleukocytosis, defined by WBC >100 000/μL, was found in 5 type A (23%) and 1 non-A type (13%) cases (p not significant, NS). According to the French–American–British classification, M4 was the most represented subtype (16 cases, 54%). Fifteen type A (68%) and 7 non-A type (87%) cases had a favorable genetic risk, whereas an intermediate risk was identified in 7 type A (32%) and 1 non-A type (13%) cases (p NS). A FLT3-ITD mutation was found in all intermediate risk patients. Thirteen (43%) patients were treated with intensive chemotherapy based on combination of antracycline and cytarabine (8 type A, 5 non-A type). A hypomethylating agent was administered to 11 (37%) patients (9 type A, 2 non-A type), while 6 (18%) patients did not received an AML directed-therapy because they died before treatment start (5 type A, 1 non-A type). Intensive chemotherapy allowed to reach a Complete Remission (CR) in 10 (77%) cases (5 type A, 5 non-A type), hypomethylating agent only in 2 (18%) cases (all type A) (p 0.048), without significant difference between the two mutation groups. Five of the 12 (42%) patients who achieved a CR experienced a relapse, in particular 3/13 (23%) cases treated with intensive chemotherapy (1 type A, 2 non-A type) and 2/11 (18%) cases who received hypomethylating agents (all type A). Molecular MRD was evaluated in 8 cases (4 type A, 4 non-A type); at the end of first line treatment it was negative for 3 patients (37%, 1 type A, 2 non-A type). All MRD negative patients received intensive chemotherapy and are still alive in CR. After a median follow-up of 31 months, median OS and DFS were 6.5 and 9 months, respectively; univariate survival data analysis using the Kaplan–Meier method showed a trend towards a statistical significance in terms of OS for non-A type cases (p 0.07, Figure 1), while DFS was not significantly different between the two groups, as a proportion of relapsed patients had a favorable outcome after salvage therapy.
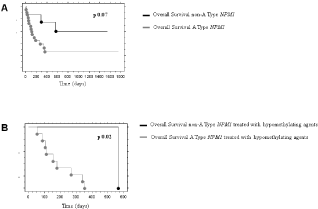
Figure 1: A: Overall survival for type A and non-A type NPM1 mutated patients; B: Overall survival for type A and non-A type NPM1 mutated patients treated with
hypomethylating agents.
When we evaluate our cohort of patients based on the therapy administered, among cases treated with hypomethylating agents OS was significantly higher for non-A type NPM1 mutated patients (p 0.02, Figure 1).
Eighteen patients (60%, 15 type A, 3 non-A type) died during the follow-up period. The cause of death was attributed mainly to AML progression (8 cases) or hemorrhagic complications (4 cases).
AML with mutated NPM1 generally portends a favorable prognosis in the absence of FLT3-ITD, although controversial data between A versus non-A type are reported. Due to the low frequency of the atypical NPM1 mutations few studies assessed their clinical relevance [4-5].
Even though in a limited number of patients, our study reports in a monocentric cohort a slightly higher incidence of NPM1 variants; about 30% of our AML patients carried an atypical NPM1 mutation compared to 20% of previously published data [5-9]. Similarly to other studies, comparable clinical and biological characteristics were found between patients with A and non-A type mutations [7- 9]. With regards to prognostic impact, we confirmed that NPM1 genotypes had no impact on obtaining a CR after induction intensive chemotherapy [7-9]. When considering OS, published results were very heterogeneous about the role of NPM1 variants [7-9]. Recently, Alpermann et al. and Heiblig et al. reported a trend towards superior OS in large cohorts of non-A type mutated patients [8-9]. These data were comparable to our findings consistent with a trend to a better OS in NPM1 variants that nevertheless require confirmation on larger patient numbers in each treatment group. At diagnosis, no difference of FLT3-ITD distribution was observed in A and non-A type mutants. Concerning other concomitant mutations, only DNMT3A mutations were linked to a worst outcome in type A and type D patients in one study [9].
Conclusion
In conclusion, our study highlights that NPM1 mutation type may have an impact on clinical outcome, in particular when hypomethylating agents are used. Nevertheless, further investigation in larger cohort of patients is needed to assess the possible role of NPM1 variants in AML risk classification, their relationship with other molecular markers and their role for treatment decisionmaking.
Acknowledgements
Fondazione Beat Leukemia Dr. Alessandro Cevenini Onlus, via Bellini 27, 20900 Monza (MB), http://www.beat-leu kemia.org, mail: info@beat-leukemia.org.
References
- Box JK, Paquet N, Adams MN. Nucleophosmin: from structure and function to disease development. BMC Mol Biol. 2016; 17: 19.
- Falini B, Mecucci C, Tiacci E. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype, N Engl J Med. 2005; 352: 254–266.
- Federici L, Falini B. Nucleophosmin mutations in acute myeloid leukemia: a tale of protein unfolding and mislocalization. Protein Sci. 2013; 22: 545–556.
- Arber DA, Orazi A, Hasserjian R. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-2405.
- Heath EM, Chan SM, Minden MD, Murphy T, Shlush LI, Schimmer AD. Biological and clinical consequences of NPM1 mutations in AML. Leukemia. 2017; 31: 798–807.
- Döhner H, Schlenk F, Habdank M. Mutant Nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005; 106: 3740–3746.
- Pastore F, Greif PA, Schneider S. The NPM1 mutation type has no impact on survival in cytogenetically normal AML. PLoS One. 2014; 9: e109759.
- Heiblig M, Sujobert P, Hayette S. Impact of NPM1 mutation subtypes on treatment outcome in AML: The Lyon-University Hospital experience. Leuk Res. 2019; 76: 29-32.
- Alpermann T, Schnittger S, Eder C. Molecular subtypes of NPM1 mutations have different clinical profiles, specific patterns of accompanying molecular mutations and varying outcomes in intermediate risk acute myeloid leukemia. Haematologica. 2016; 101: e55-58.
- Döhner H, Estey E, Grimwade D. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129: 424-447.