
Case Report
Ann Hematol Oncol. 2021; 8(4): 1338.
A Multirefractory Lymphomatoid Granulomatosis Successfully Treated with Gemcitabine as a Single Agent: Case Report and Review of the Literature
Ku J¹*, Bron D¹, Wind RD², Meuleman N¹ and Massaro F¹
¹Department of Hematology, Bordet Institute, Universite Libre de Bruxelles, Belgium
²Department of Anatomopathology, Bordet Institute, Universite Libre de Bruxelles, Belgium
*Corresponding author: Ku JK, Department of Hematology, Bordet Institute, Boulevard de Waterloo 121, 1000 Bruxelles, Belgium
Received: February 22, 2021; Accepted: March 22, 2021; Published: March 29, 2021
Abstract
Lymphomatoid Granulomatosis (LYG) is a rare, EBV-driven disease with angiocentric and angiodestructive pleomorphic lymphocytic infiltrates. Pulmonary involvement is frequent and responsible for pulmonary manifestations which may be associated with systemic symptoms. Its rarity makes it difficult to diagnose, and the diagnosis is usually made after several months of investigation. There is no consensus on treatment, which can range from a waitand- see approach to multidrug therapy with allogeneic hematopoietic stem cell transplantation, according to histological grade. The lack of consensus does not help in the management of these patients. We report the case of a patient with a multirefractory LYG, who achieved a Complete Remission (CR) with gemcitabine as single agent salvage treatment.
Keywords: Lymphomatoid granulomatosis; Non Hodgkin lymphoma; Rare lymphoma; Lymphoproliferative disorder; Autologous stem cell transplantation
Introduction
LYG was first described by Liebow AA in 1972 [1]. It was introduced into the WHO classification of hematological tumors and lymphoid tissues in 2001 and the 2016 revision describes it as a mature B-cell neoplasm [2]. It is a very rare entity, counting for <1% of non Hodgkin’s lymphoma, with approximately 600 cases reported in the literature, which affects men more than women (H:F ratio >2:1), in adulthood with a mean age of 48 years and is related to Epstein-Barr Virus (EBV) and therefore more likely to affect immunocompromised subjects, such as patient suffering from HIV, congenital immunodeficiency or with hematopoietic stem cell transplantation. Pulmonary involvement is cardinal, being present in approximately 90-100 % of cases [1-5].
Morphological examination reveals an angiocentric and angiodestructive polymorphic lymphoid infiltrate. Granulomatosis is an inconstant finding. The histological grade depends on the degree of EBV expression assessed by In Situ Hybridization (ISH). A higher expression determines a higher grade, correlating with disease aggressivity. It is especially important to distinguish grade 1-2 (<5 EBER+/hpf, 5-20 EBER+/hpf) from high grade, grade 3 (> 50 EBER+/ hpf) as this will determine different therapeutic approach [2].
In immunophenotypic study, atypical B cells mark CD20, CD30 may be positive, but CD15 is negative. Monoclonality can be demonstrated in high grades by immunoglobulin chains rearrangement [2,4]. Because of the very rare occurrence of the entity, there is no consensus concerning standard treatment: experts recommendations are primarily based on data from case reports and retrospective series [3].
Clinical Case
Our patient is a 60-year-old Caucasian man, who consulted for chest pain associated with hemoptysis, an involuntary weight loss of 20kg and asthenia. The patient is a construction worker presenting several comorbidities: arterial hypertension, type 2 diabetes mellitus, chronic obstructive gold III pulmonary disease and thromboembolic disease without an identified contributing factor treated 2 years earlier. Family history was unremarkable. His treatment consisted of an ACE inhibitor, a beta blocker and an oral hypoglycemic agent. Clinical examination reveals a decreased vesicular murmur on the right lung field at pulmonary auscultation.
A Computed Tomography (CT) of the chest shows an excavated mass in the upper right lobe of 7×4×5 cm with a central bell of 4×4×2 cm, areas of postero-basal lower right lobe consolidation and a centimetric right hilar adenomegaly, as well as a left paramediastinal mass of 6×6×6 cm at the level of the esogastric junction. The 18F-FDG - Positron Emission Tomography (PET) CT-scan assessment reveals hypermetabolic areas in the voluminous right lung mass (SUV max 15.12), pulmonary nodules (SUV max 13.09), necrotic-looking masses at the level of the diaphragmatic pillars (SUV max 11.23) and several hypermetabolic hepatic lesions (SUV max 14) (Figure 1).
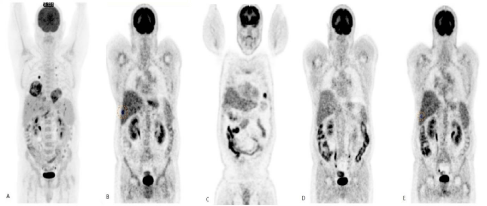
Figure 1: A: Pretreatment pet CT showing hypermetabolic activity in the voluminous right lung mass (SUV max 15.12), pulmonary nodules (SUV max 13.09),
necrotic-looking masses at the level of the diaphragmatic pillars (SUV max 11.23) and several hypermetabolic hepatic lesions (SUV max 14). B: Early relapsed with
two hypermetabolic hepatic lesions (SUV max 7.2). C: Partial response at the hepatic level but new pulmonary lesions after salvage therapy with ICE and Interferon
alpha. D: Complete remission after gemcitabine as single agent therapy. E: Confirmation of a complete remission after consolidation with BEAM conditioning
regimen followed by autologous stem cells transplantation.
Note: Intestinal capitation in the context of oral hypoglycemic agent.
Blood cell counts shows bicytopenia with microcytic anemia at 11g/dL, absolute lymphopenia at 850/μL with a normal leucocytes count, a normal platelet count, LDH level within the norms of 165IU/L, an inflammatory syndrome with a CRP level of 82mg/L, an increased ESR at 83mm/h, and beta 2 microglobulin at 3.4mg/L.
An infectious workout reveals the presence of EBV IgG without IgM, absence of HIV infection; a negative Quantiferon test. Aspergillus antigen and the culture test for Koch’s bacilli are negative in sputum and Bronchioloalveolar Lavage (BAL). A transbronchial lung biopsy is performed, showing a proliferation of atypical large cells, destroying the pulmonary architecture, with lymphomatous features.
Immunostaining is positive for CD20, CD79A, and CD30 and negative for CD15 and CD10. On a molecular level, BCL2, CMYC, MUM1 and BCL6 are negative. Value of Ki67 expression is 90%. Epstein-Barr Encoding Region (EBER) FISH probe shows a strong expression (EBV grade 3). Numerous CD68+ histiocytes as well as a T CD3+ CD5+ population are found. Liver masses are also biopsied; revealing lymphomatous infiltration (EBV grade 1). The diagnosis is consistent with a lymphomatoid granulomatosis of high grade (EBV grade 3). The patient presents a stage IVb according to Ann Arbor’s classification.
The patient has been treated with 6 cycles of R-CHOP 21 (rituximab375 mg/m², cyclophosphamide 750 mg/m², adriamycin 50mg/m2, vincristine 1.4mg/m², prednisolone 40mg/m²) and two additional administration of rituximab. The end-of-treatment 18F-FDG-PET/CT shows a Complete Metabolic Remission (CMR). However, two months later, the patient has relapsed with two hypermetabolic hepatic lesions on 18F-FDG-PET/CT (SUV max 7.2) (Figure 1). The biopsy confirms the relapsed low-grade (EBV grade 1) lymphomatoid granulomatosis.
A salvage treatment with ICE (Ifosfamide 5g/m², Carboplatin AUC 5mg/mL/min, Etoposide 100mg/m²) is administered: after two cycles, the 18F-FDG-PET/CT demonstrates disease progression. A third line of treatment with a combination of prednisolone and interferon alpha using gradually increased doses (up to 5 million units, 3 times for week) is administered with a mixed response (partial response at the hepatic level but occurrence of lung lesions) (Figure 1). Finally, a fourth line treatment with gemcitabine (1000mg/m², d1 and d15 in 28 day cycles) is started. An 18F-FDG-PET/CT after 3 cycles shows the achievement of a CMR. A consolidation strategy with autologous stem cells transplantation (auto-SCT) conditioned with BEAM chemotherapy is then applied. The patient is currently in CMR 12 months after the auto-SCT.
Discussion
LYG is a heterogeneous disease, with a heterogeneous presentation in terms of aggressivity. However, several old retrospective series reported poor outcomes in aggressive diseases, with a median survival of 14 months [1,4]. The main differential diagnoses have to be made between infectious causes, granulomatosis with polyangiitis, necrotizing sarcoidosis, benign granulomatosis, and lymphocytic angiitis [5]. Because of the rare occurrence of the disease, no standard treatment is defined. However, recent expert recommendations based on data from case reports and retrospective series have been published [3].
Treatment of low-grade diseases
LYG preferentially affects persons with an underlying immune deficiency [2,6]. Low-grade diseases represent 50% of the cases and are usually polyclonal and thus less aggressive. In these cases, it is recommended, when possible, to reduce or interrupt the immunosuppressive treatment, a strategy that can lead to spontaneous remissions [4,6-8]. It has to be noted that the strategy is similar than the management strategy for PTLD [9]. If not possible or ineffective, Wilson et al. reported 4 cases of low-grade LYG treated with alpha interferon both in first and second line, with achievement of complete and durable responses [10].
Treatment of high-grade diseases
The monoclonality of high-grade diseases is associated to increased clinical aggressiveness and poorer prognosis. The treatment is thus more aggressive and based on polychemotherapy regimens. However, few comparative studies are available, likely due to disease rarity, with the majority of available results deriving from small retrospective reports [11-18]. Treatment regimens are heterogeneous and the choice of the regimen depends on the clinical setting as well as patient fitness.
A commonly used regimen is R-CHOP, but it has not been prospectively studied so far. However, since the disease is close to Diffuse Large B Cell Lymphoma (DLBCL) and more particularly EBV-positive DLBCL, R-CHOP is believed to be effective. Castillo provided an update in 2016 regarding treatments for EBV-positive DLCBL in elderly patients. He found that EBV-positive DLBCL had a lower rate of response to combination chemotherapies comparing to EBV-negative DLBCL, but the addition of immunotherapy, specifically rituximab, provided a better response rate with OR rates of 50-90% and CR rates of 30-70 % [19].
Chavez has published a retrospective review of 11 cases of LYG treated at his institution. R-CHOP was the most frequently used regimen with 5/11 patients treated with it. Considering all mixed treatments, the Overall Response Rate (ORR) was 63.6% and 36.4% achieved Complete Remission (CR) [20].
The R-DA-EPOCH regimen alternating with interferon alpha therapy has been evaluated in a prospective study: the 5-year PFS and OS were 41% and 70.8%, respectively [21]. However, it should be noted that the median age for this study is 46.2 years and that the R-DA-EPOCH regimen is unlikely to be applicable to older patients for the elevated toxicity associated.
Treatment of refractory or recurrent disease
Relapse is a frequent event in LYG despite CMR achievement at the end of first-line treatment. This is probably related to the fact that the disease is related to a state of immunosuppression which is not resolved by the treatment. However, few experiences in literature have explored recurrence treatment. It should be noted that relapses may present a different histologic grade from the initial presentation and therefore the treatment regimen depends on it. In the event of low-grade recurrence, treatment with IFN alpha has been reported to be successful [10]. High-grade recurrences are treated with polychemotherapy regimens. Since LYG is a mature B-lineage neoplastic entity, active regimens for the management of recurrent high-grade B-lymphomas have been widely applied to this population. Due to the presence of a vast variety of chemotherapy regimens, the choice strongly relies on center experience, patient’s comorbidities and age [22]. A frequently suggested regimen is R-GEMOX, which employs gemcitabine, an active agent in B-lymphoproliferative diseases. Data for gemictabine as single agent therapy failed to show good response rates, with 0% CR, 19% ORR, 36% stable disease, and 42% progressive disease, but the toxicity of the therapy is generally low which makes it a well-tolerated treatment [23]. Anecdotally, Friedrichs and Thiel reported a case of a 36 year old woman suffering from recurrent LYG who achieved complete remission with radioimmunotherapy treatment [24].
Concerning the benefit of treatment intensification, autologous and allogeneic Stem Cell Transplantation (SCT) have also been employed with mixed results [21-23]. Siegloch performed a review of auto- and allo- SCT cases reported in EBMT registries: after a median follow-up of 5.1 years, 6 of the 10 reported patients were still alive [25]. This small series does not allow conclusions related to the benefit of intensification. This strategy should be discussed on a caseby- case basis and reserved to fit patients.
Our patient presented an early hepatic relapse after R-CHOP treatment. Several salvage treatments, both with chemotherapy (ICE), and immunotherapy (interferon alpha) were unsuccessful. Interferon alpha presented a good activity on hepatic lesions (characterized as histological low-grade disease) but was unable to confer a good control of the pulmonary disease. We therefore decided to treat the patient as a mature DLBCL. Since prognosis is known to be poor in multirefractory LYG, we first proposed gemcitabine as single agent because the patient had already received a platinumbased regimen and presented an early relapse after exposure to an anti-CD20 monoclonal antibody. The patient achieved CMR and we decided to consolidate the treatment with an autologous SCT. To our knowledge, this is the first case of LYG treated with gemcitabine who achieved complete remission before autografting.
Conclusion
LYG is an extremely rare lymphoma, frequently associated with a poor outcome because of the absence of curative treatments and the high relapse rate. Many therapeutic regimens have been proposed, but the absence of prospective studies and the small number of enrolled patients does not allow drawing any conclusions. We reported a CR in a multirefractory patient using a salvage treatment with gemcitabine as a single agent. This drug, which is already employed in other lymphoproliferative disorders, could represent a safe and effective option also in the LYG, especially in relapsed/refractory setting.
References
- Liebow AA, Carrington CR, Friedman PJ. Lymphomatoid granulomatosis. Hum Pathol. 1972; 3: 457-558.
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-2390.
- Melani C, Jaffe ES, Wilson WH. Pathobiology and treatment of lymphomatoid granulomatosis, a rare EBV-driven disorder. Blood. 2020; 135: 1344-1352.
- Katzenstein AL, Carrington CB, Liebow AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979; 43: 360-373.
- Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002; 20: 750-762.
- Yang SH, Yeh YC, Chan YJ, Chou TY, Hsiao LT. A solitary pulmonary lymphomatoid granulomatosis-like lesion as the only manifestation of post-transplant lymphoproliferative disorder after hematopoietic stem-cell transplantation. Ann Hematol. 2011; 90: 1481-1483.
- Blanchart K, Paciencia M, Seguin A, Chantepie S, Du Cheyron D, Charbonneau P, et al. Fatal pulmonary lymphomatoid granulomatosis in a patient taking methotrexate for rheumatoid arthritis. Minerva Anestesiol. 2014; 80: 119-120.
- Aiko N, Sekine A, Umeda S, Katano T, Matama G, Isomoto K, et al. The Spontaneous Regression of Grade 3 Methotrexate-related Lymphomatoid Granulomatosis: A Case Report and Literature Review. Intern Med. 2018; 57: 3163-3167.
- Heiner Zimmermann, Ralf U. Trappe; EBV and post transplantation lymphoproliferative disease: what to do? Hematology Am Soc Hematol Educ Program. 2013; 2013: 95-102.
- Wilson WH, Kingma DW, Raffeld M, Wittes RE. Association of Lymphomatoid Granulomatosis with Epstein-Barr Viral Infection of B Lymphocytes and Response to Interferon-alpha 2b. Blood. 1996; 87: 4531-4537.
- Bartosik W, Raza A, Kalimuthu S, Fabre A. Pulmonary lymphomatoid granulomatosis mimicking lung cancer. Interact Cardiovasc Thorac Surg. 2012; 14: 662-664.
- Gorospe L, Calleja-Lopez JL, Benito-Berlinches A. Pulmonary lymphomatoid granulomatosis diagnosed by image-guided core needle biopsy. Asian Cardiovasc Thorac Ann. 2018; 26: 70-71.
- Jung KH, Sung HJ, Lee JH, Lee KY, Shin JS, Kim KM, et al. A case of pulmonary lymphomatoid granulomatosis successfully treated by combination chemotherapy with rituximab. Chemotherapy. 2009; 55: 386-390.
- Lee S, Kang MJ, Kim HJ, Kim HB, Lee J. Pulmonary lymphomatoid granulomatosis in a 4-year-old girl. Pediatr Radiol. 2015; 45: 1082-1085.
- Xu B, Liu H, Wang B, Zhang H, Wu H, Jin R, et al. Fever, Dry Cough and Exertional Dyspnea: Pulmonary Lymphomatoid Granulomatosis Masquerading as Pneumonia, Granulomatosis with Polyangiitis and Infectious Mononucleosis. Intern Med Tokyo Jpn. 2015; 54: 3045-3049.
- Xu L, Zhang X, Lu YJ, Zheng YH, Gao GX. Pulmonary Lymphomatoid Granulomatosis with Hemophagocytic Lymphohistiocytosis as the Initial Manifestation. Front Oncol. 2020; 10: 34.
- Culhaci N, Levi E, Sen S, Kacar F, Meteoglu I. Pulmonary lymphomatoid granulomatosis evolving to large cell lymphoma in the skin. Pathol Oncol Res POR. 2002; 8: 280-282.
- Robak T, Kordek R, Urbanska-Rys H, Robak P, Bartkowiak J, Bednarek A, et al. High activity of rituximab combined with cladribine and cyclophosphamide in a patient with pulmonary lymphomatoid granulomatosis and bone marrow involvement. Leuk Lymphoma. 2006; 47: 1667-1669.
- Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management: EBV+ DLBCL 2016 update. Am J Hematol. 2016; 91: 529-537.
- Chavez JC, Sandoval-Sus J, Horna P, Dalia S, Bello C, Chevernick P, et al. Lymphomatoid Granulomatosis: A Single Institution Experience and Review of the Literature. Clin Lymphoma Myeloma Leuk. 2016; 16: S170-S174.
- Melani C, Roschewski M, Pittaluga S, Cohen J, Lucas AN, Steinberg SM, et al. Phase II Study of Interferon-Alpha and DA-EPOCH+/-R in Lymphomatoid Granulomatosis. Blood. 2018; 132: 785-785.
- Gisselbrecht C, Van Den Neste E. How I manage patients with relapsed/ refractory diffuse large B cell lymphoma. Br J Haematol. 2018; 182: 633-643.
- Fosså A, Santoro A, Hiddemann W, Truemper L, Niederle N, Buksmaui S, et al. Gemcitabine as a Single Agent in the Treatment of Relapsed or Refractory Aggressive Non-Hodgkin’s Lymphoma. J Clin Oncol. 1999; 17: 3786-3792.
- Friedrichs B, Thiel E. Relapsed pulmonary lymphomatoid granulomatosis grade III: curative treatment with radioimmune therapy and autologous stem cell transplantation. Dtsch Med Wochenschr. 2010; 135: 1857-1860.
- Siegloch K, Schmitz N, Wu HS, Friedrichs B, van Imhoff GW, Montoto S, et al. Hematopoietic Stem Cell Transplantation in Patients with Lymphomatoid Granulomatosis: A European Group for Blood and Marrow Transplantation Report. Biol Blood Marrow Transplant. 2013; 19: 1522-1525.