
Case Report
Ann Hematol Oncol. 2021; 8(7): 1352
Off-Pump HeartMate 3® LVAD Implantation via Left Thoracotomy to Descending Aorta: Transition from Transaxillary Impella 5.0® LVAD
Luciano R¹, Arce A², Tuluca A², Bozorgnia B², Bonita R², Banka S², Hamshari YA², Robbins T², Mossayebi MH² and Samuels L³*
1Stockton University, Galloway, NJ, USA
2Departsments of Cardiothoracic Surgery and Cardiology, Albert Einstein Medical Center, Philadelphia, PA, USA
2Departsment of Cardiothoracic Surgery, Thomas Jefferson University, Philadelphia, PA, USA
*Corresponding author: Louis Samuels, Department of Cardiothoracic Surgery, Thomas Jefferson University, Philadelphia, PA, USA
Received: April 11, 2021; Accepted: May 17, 2021; Published: May 24, 2021
Abstract
A 62-year-old man with a prior history of Coronary Artery Bypass Grafting (CABG) presented in cardiogenic shock. A percutaneous left femoral Impella CP® Left Ventricular Assist Device (LVAD) was placed with modest improvement in hemodynamics. The LVAD was upgraded to an open right transaxillary Impella 5.0® with hemodynamic stabilization. Cardiacfunction was assessed with serial echocardiography demonstrating persistent severe left ventricular dysfunction. In view of previous CABG with patent Left Internal Mammary Artery (LIMA) graft the decision was made to place a HeartMate 3® LVAD via left thoracotomy with LV apical inflow and descending aortic outflow. This approach was completed without the need for Cardiopulmonary Bypass (CPB). The postoperative course was uneventful and discharge to a rehabilitation center occurred on the ninth postoperative day.
Keywords: Coronary Artery Bypass Grafting (CABG); Left Ventricular Assist Device (LVAD); Cardiogenic shock
Introduction
Among the indications for the use of a Left Ventricular Assist Device (LVAD) is Cardiogenic Shock (CS). LVADs may be shortterm (days to weeks), intermediate-term (weeks to months), or longterm (months to years). Short and intermediate term LVADs tend to be utilized as a Bridge-to-Recovery (BTR). Long-term devices are used as a Bridge-to-Transplant (BTT) or Destination Therapy (DT). Patients presenting in acute cardiogenic shock may or may not recover native heart function. As such, multiple LVADs may be necessary to maintain hemodynamic support until a final endpoint is determined.
The incidence of cardiogenic shock following an acute Myocardial Infarction (MI) ranges between 5% and 15% with an average of 7.5% [1]. The mortality associated with acute MI with CS approaches 80%, mirroring the mortality associated with post-cardiotomy shock requiring multiple high dose inotropes [2]. The treatment options for acute MI-CS ranges from intravenous vasopressors/ inotropes to Extra-Corporeal Membrane Oxygenation (ECMO) with theuse of short-term LVAD support as a bridge to recovery, bridge to transplant, or bridge to a long-term device. The purpose of this paper is to describe a complex case of acute MI-CS in which several LVAD technologies were utilized to minimize surgical risk and maximize hemodynamic support. The transition from a shortterm Impella® (Abiomed, Inc., Danvers, MA, USA) to a long-term HeartMate 3® (Abbott Laboratories, Abbott Park, IL, USA) LVAD via left thoracotomy to descending aorta without the need for cardiopulmonary bypass is a novel approach for this complex case.
Case Presentation
A 62-year-old-male presented with shortness of breath and chest pain for 5 days prior to admission. His past medical history consisted of Coronary Artery Disease (CAD), Coronary Artery Bypass Grafting (CABG), active tobacco use, and type 2 diabetes. Upon arrival to the emergency room, he was tachypneic, tachycardic, and hypoxic. His extremities were cool to the touch. A chest CT was obtained showing pulmonary edema. The EKG showed ST depressions in anterior and inferior leads with T wave inversions and ST elevations in lead I and aVL. Echocardiography demonstrated 30% (EF) with hypokinesis at the apex and septum. He was taken to the cardiac catheterization lab where he Ejection Fraction required intubation, defibrillation, chest compressions and chemical resuscitation. Percutaneous placement of an Impella CP® via the left femoral artery was necessary. Coronary angiography revealed diffuse multi-vessel CAD not amenable to Percutaneous Coronary Intervention (PCI) or CABG. Although marginally stabilized, it was determined that the Impella CP® was not sufficient to maintain optimal hemodynamics. A right transaxillary Impella 5.0® was placed in the hybrid catheterization lab together with interventional cardiology. Once stable, the Impella CP® was removed from the left femoral artery. Serial echocardiography over the next two weeks demonstrated persistent Left Ventricular (LV) dysfunction with Impella 5.0® dependence. The decision was made to transition to the HeartMate 3® implantable LVAD. In view of the previous CABG surgery with a patent LIMA graft, an alternative implant approach was utilized.
Device
Incision(S)
Inflow
Outflow
CPB
Reference
BVS5000
LT/HEMISTERNOTOMY
LTPULM.V.
DESC.AORTA
NO
3
HVAD
LT/HEMISTERNOTOMY
LV
ASC.AORTA
YES
4
HVAD
LT
LV
DESC.AORTA
YES
5
HVAD
LEFT SUBCOSTAL
LV
ABD.AORTA
YES
6
HVAD
STERNOTOMY
LV
LT. INOMINATE A.
YES
6
HVAD
STERNOTOMY
LV
LT. SUBCL.A.
YES
6
HM2
LT/HEMISTERNOTOMY
LV
ASC.AORT
YES
7
HM2
LT/HEMISTERNOTOMY
LV
ASC.AORTA
YES
8
HM3
LT/RTPARASTERNAL
LV
ASC.AORTA
YES
9
HVAD
LT/HEMISTERNOTOMY
LV
ASC.AORTA
YES
10
HVAD/HM2
LT
LV
DESC.AORTA
YES
11
HM2
LT
LV
DESC.AORTA
NO
12
HVAD
LT
LV
DESC.AORTA
YES*
12
HVAD
LT
LV
DESC.AORTA
NO
13
HM3
LT/RT PARASTERNAL
LV
ASC.AORTA
NO^
14
HM3
LT
LV
DESC. AORTA
YES
15
HM3
LT
LV
DESC.AORTA
NO
THIS CASE
HVAD: HeartWare®; HM: HeartMate®: LT: Left Thoracotomy; PULM: Pulmonary; SUBCL: Subclavian; *Use of Adenosine for temporary cardiacstand still while coring LVapex and insertingLVAD; ^Use of rapid pacing while coring LV apex and inserting LVAD.
Table 1: Summary of reports with alternative LVAD implant techniques.
The patient was brought to the operating room (OR) and positioned for entry into the left chest. A left anterolateral thoracotomy incision was made at the 5th intercostal space. Single lung ventilation was utilized and well tolerated. The inferior pulmonary ligament was divided up to the level of the inferior pulmonary vein to expose the descending aorta. Heparin was administered and a partial occluding clamp placed on the aorta (Figure 1a). The outflow graft was anastomosed end-to-side noting the length of the graft to the LV apex. The LV apical inflow insertion site was identified by palpation and Transesophageal Echocardiographic (TEE) guidance. The HeartMate 3® sewing ring was chosen and positioned at the optimal location. The pump was brought to the field and oriented to attach the outflow graft without distortion by the lung. The driveline was tunneled to an appropriate sub cost allocation. The Impella 5-0® was withdrawn through the axillary artery under TEE guidance. At this point the decision was made to continue without Cardiopulmonary Bypass (CPB) cannulation. The LV apex was cored, and the HeartMate 3® pump was inserted into the apex of the LV and secured to these wing ring (Figure 1b). A clamp was placed across the outflow graft, the pump initiated, and the vent catheter placed proximal to the clamp for de-airing. The inflow cannula was inspected via TEE and positioned to face the mitral valve. The lung was inflated, the TEE inspected for adequate de-airing, and the cross-clamp removed from the outflow graft. Pump speed was optimized followed by closure of the incision. The patient was recovered in the ICU with a satisfactory post-operative chest x-ray (Figure 2).
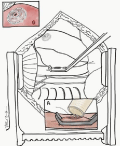
Figure 1a: Illustration of HM3® LVAD placement from LV apex to descending
aorta; lung retracted cephalad with dissection of inferior pulmonary ligament
for optimal exposure.

Figure 1b: Illustration of HM3® LVAD placement from LV apex to descending
aorta; lung retracted cephalad with dissection of inferior pulmonary ligament
for optimal exposure.
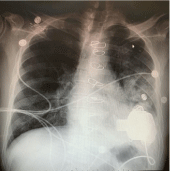
Figure 2: Post-Op Chest Radiograph demonstrating orientation of HM3®.
Discussion
The use of LVADs for acute and chronic heart failure has evolved considerably since their first debut in the 1960s. The devices at that time were large, complex, and with unpredictable durability. As the technology improved and their interaction in the human body better understood, it was only natural that their application expanded to a larger patient population that suffered from either acute CS or Chronic Heart Failure (CHF).
At present, the state-of-the-art technologies fall into two types: percutaneous LVADs for acute CS and implantable LVADs for CHF. Interestingly, both types of technology are continuous flow pumps, an engineering design that simplified the mechanics of blood flow, extended durability, and reduced device size. These features expanded the application of the pumps to a broader patient population including pediatric and young adults with smaller body surface areas.
The next evolution in LVAD application was the use of alternative surgical implantation techniques. As the complexity of patients requiring LVADs increased, the traditional approach-LV apical inflow with ascending aortic outflow via sternotomy-was notal ways safe or feasible, particularly in those patients with one or more previous transsternal open-heart procedures. As such, creative implants techniques have been utilized [3-15]. The case described in this report represents the use of several LVAD technologies in various locations to treat both acute and chronic conditions. Initially, the patient presented in cardiogenic shock that required rapid deployment of a percutaneous Impella CP® placed in the left femoral artery. Upgrade to an Impella 5.0® was necessary to optimize hemodynamic stability. Finally, transition to a HeartMate 3® implantable LVAD was performed due to failure to wean from the Impella 5.0® after several weeks.
The surgical technique for the HeartMate 3® LVAD is noteworthy in this case. In view of the patient’s previous CABG surgery with a patent LIMA graft situated directly under the sternum, the decision was made to implant the HeartMate 3® device via left thoracotomy with inflow from the LV apex and outflow to the descending aorta. The decision to use this approach is not novel, having been performed previously with other devices [5,7,11-15]. In these reports, either the HVAD® (HeartWare, Inc. Framingham, MA) or the HeartMate 2® (Abbott Laboratories, Abbott Park, IL) was utilized with the majority placed on-pump via femoral cannulation. In one report by Maltais et al., an HVAD® was placed via left thoracotomy with outflow to the descending aorta off-pump using boluses of adenosine to temporarily induce cardiac standstill [12]. Kocabeyoglu et al. implanted a HeartMate 3® via left thoracotomy to descending aorta with the aid of CPB [15]. To our knowledge, our case is the first to report implantation of a HeartMate 3® via a left thoracotomy to the descending aorta off-pump without the aid of temporary chemical arrest or rapid ventricular pacing [12,14]. The notable aspects of the case include two experienced LVAD surgeons for the implant, similar to the approach described by Potapov et al. [14]. An important moment in the offpump technique occurs when the coring is performed and the LVAD is inserted. The potential for excessive bleeding is not to be under estimated. Experience with LVAD exchange performed off-pump was valuable in understanding the sequence of the LVAD insertion into the LV. The use of adenosine or rapid ventricular pacing, as described in previous reports [12,14], introduces the potential for hemodynamic instability during the implant and following it. As such, we deferred the use of these techniques. Instead, we preferred deployment of the LVAD following LV apical coring. It is important to note that the sewing ring was applied prior to coring in order to allow for rapid insertion of the LVAD inflow with minimal bleeding and air entry. It was observed with this technique that there was no LV apical air on TEE, an advantage in terms of avoiding air embolization to the coronary arteries as well as the cerebral circulation.
In summary, we report the implantation of a HeartMate 3® LVAD performed off-pump via left thoracotomy with outflow to the descending aorta. We believe this to be a useful technique for cases in which the traditional approach is hazardous.
References
- Goldberg RJ, Gore JM, Alpert JS, et al. Cardiogenic shock after acute myocardialinfarction-incidence and mortality from acommunity-wide perspective, 1975 to 1988. NEngl J Med. 1991; 325: 1117-1122.
- Samuels LE, Kaufman MS, Thomas, MP, et al. Pharmacological criteria for ventricular assistdevice insertion following postcardiotomy shock: experience with the Abiomed BVS system. J Card Surg. 1999; 14: 288-293.
- Samuels LE, Thomas MP, Morris RJ, et al. Alternative sites for Abiomed BVS 5000 left ventricular assist device implantation. Journal of congestive heart failure and circulatory support. 1999; 1: 85-89.
- Schmitto JD, Molitoris U, Haverich A, et al. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: Upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg. 2012; 143: 511-513.
- Umakanthan R, Haglund NA, John M, Stulak JM, et al. Left Thoracotomy Heart Ware Implantation with Outflow Graft Anastomosis to the Descending Aorta: A Simplified Bridge for Patients With Multiple Previous Sternotomies. ASAIO J. 2013; 59: 664-667.
- El-Sayed Ahmed MM, Aftab M, Singh SK, Hari R. Mallidi, et al. Left ventricular assist device out flow graft: alternative sites. Ann Cardiothorac Surg. 2014; 3: 541-545.
- Cho YH, Deo SV, Schirger JA, et al. Implantation of a HeartMate II Left Ventricular Assist Device via Left Thoracotomy. Ann Thorac Surg. 2012; 94: 1712-1714.
- Samuels LE, Casanova-Ghosh E, Rodriguez R, et al. Left ventricular assist device implantation in high risk destination therapy patients: an alternative surgical approach. Journal of Cardiothoracic Surgery. 2012; 7: 21.
- Wood KL, Ayers BC, Sagebin F. Complete Sternal-Sparing HeartMate 3 Implantation: A Case Series of 10 Consecutive Patients. AnnThorac Surg. 2019; 107: 1160-1165.
- Schmitto JD, Molitoris U, Haverich A. Implantation of a centrifugal pump as a left ventricular assist device through a novel, minimized approach: Upper hemisternotomy combined with anterolateral thoracotomy. J Thorac Cardiovasc Surg. 2012; 143: 511-513.
- Mueller LM, Cho SH, Fritz T, et al. Left Ventricular Assist Device Placement through Left Thoracotomy in Difficult Scenarios. Int J Clin Cardiol. 2016; 3: 69.
- Maltais S, Davis ME, Haglund N, et al. Minimally invasive and alternative approaches for long-term LVAD placement: the Vanderbilt strategy. Ann Cardiothorac Surg. 2014; 3: 563-569.
- Ozbaran M, YagdI T, Engin C, et al. Left ventricular assist device implantation with left lateral thoracotomy with anastomosis to the descending aorta. Interactive Cardio Vascular and Thoracic Surgery. 2018; 27: 186-190.
- Potapov EV, Kukucka M, Falk V, et al. Off-pump implantation of the HeartMate 3 left ventricular assist device through bilateral thoracotomy approach. J Thorac Cardiovasc Surg. 2017; 153: 104-105.
- Kocabeyoglu SS, Kervan U, Sert DE, et al. Heartmate 3 implantation via left thoracotomy with descending aortic anastomosis. J Card Surg. 2019; 34: 635-637.