
Case Report
Ann Hematol Oncol. 2021; 8(7): 1356.
Subcutaneous Tranexamic Acid: A Novel Approach to Managing Bleeding
Sutherland A¹*, Carey M¹ and Miller M²
¹Sir Michael Sobell House Hospice, Churchill Hospital, England
²Department of Medicine, Oxford University, England
*Corresponding author: Anna Sutherland, Sir Michael Sobell House Hospice, Churchill Hospital, Old Road, Oxford OX3 7LE, England
Received: April 26, 2021; Accepted: May 26, 2021; Published: June 02, 2021
Abstract
We describe the case of a 68 year old with a transglottic squamous cell carcinoma, a tracheostomy and persistent blood stained tracheal secretions. Oral and intravenous Tranexamic Acid (TA) effectively controlled the bleeding. On losing both routes, we administered 2g of TA (20ml) by continuous subcutaneous infusion over 24 hours. Control of bleeding was maintained over 18 days until death. No site reactions were observed.
A literature review was undertaken, however, none of the studies looked at the use of TA in an end of life or palliative care population. We identified 3 clinical palliative care guidelines relating to continuous subcutaneous administration of TA. Further use should be reported in the literature to build the evidence base surrounding this novel practice.
Keywords: Other pharmacology and therapeutics; Pharmacology and therapeutics Clinical; Other palliative care; Palliative care Clinical; Hospice; Palliative care
Background
The use of enteral and intravenous Tranexamic Acid (TA) to manage chronic bleeding at end of life is well established. The subcutaneous administration of TA is an off-label route of administration of a licenced drug, not previously reported in the literature, offering ongoing control of bleeding in the last days of life.
Case Presentation
We describe the case of a 68 year old with a transglottic squamous cell carcinoma, a tracheostomy and persistent blood stained tracheal secretions. Severe recurrent bleeding resulted in a prolonged hospital admission. Bleeding was initially managed successfully with oral TA (1g three times a day). On developing a tracheo-oesophageal fistula the oral route was lost, TA was administered intravenously. However, after a few days intravenous cannulation became increasingly challenging.
A continuous subcutaneous infusion of TA (2g/24 hours) was administered via a T34 McKinley syringe driver, made up to 21mls with water for injection. The dose was unchanged over the subsequent 18 days until the patient died. No other medications were mixed with the TA infusion. Bleeding remained well controlled until death.
There were no subcutaneous site reactions noted over the course of 18 days. On a single occasion, the syringe contents were noted to be cloudy, necessitating discarding of the contents and making up a new infusion.
Literature Search
Medline, EMBASE and CINAHL were searched on 2nd of July 2020. The search strategy used [“subcutaneous” AND “tranexamic acid” without language restriction, covered use in adults and children, all study types, published and unpublished records.
96 records were identified (Figure 1). None of the studies looked at the use of TA in an end of life or palliative care population.
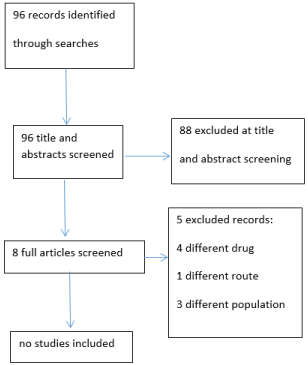
Figure 1: PRISMA diagram.
Clinical Guidelines
We identified 3 unpublished palliative care guidelines relating to continuous subcutaneous administration of TA [1-3].
Two guidelines suggested use of a subcutaneous infusion of TA if there is loss of the oral route and anticipated benefit from ongoing therapy [1,2] or, the third adds, patient ( or family) fear of recurring bleeding [3]. A dose of 1500–2000 mg/24 hours is suggested using water for injection as a diluent [1-3]. An oral to parenteral conversion ratio of 1:1 is postulated [1,2]. It is not recommended that TA is combined with other medications in the infusion [2,3]. The product is noted to have a neutral pH 6.5- 8.0 [1,2].
Discussion
To the best of our knowledge, we report the first published case of tranexamic acid administered via a continuous subcutaneous infusion.
Three published surgical trials report the bolus administration of TA subcutaneously during surgery [6-8]. A total of 188 participants received a single dose of placebo or TA, mixed with lidocaine intraoperatively during skin cancer surgery, facelift and blephoroplasty. A single adverse reaction was reported of erythema and local oedema resolving within 2 hours [7].
The identified clinical guidelines suggest a body of palliative medical experience of off-label administration of subcutaneous infusions in both hospice and home settings. From first principles, this appears to be a pragmatic approach in an unusual clinical situation. TA seems unlikely to cause troublesome site reactions given the neutral pH of the solution. The guidelines raise the potential for subcutaneous TA administration at home, facilitating discharge.
In the absence of a literature base to support practice the authors recommend that future use of subcutaneous TA at the end of life is reported in the literature.
Conclusion
We report a case of off-label subcutaneous tranexamic acid administration via continuous infusion over 18 days to successfully manage bleeding from a transglottic cancer when all licenced routes of administration were lost. Further use should be reported in the literature to build the evidence base surrounding this novel practice.
Contributorship Statement
AS identified the case and drafted the manuscript, undertaking the literature review. MC and MM had supervising author input throughout the drafting the final paper. manuscript.
Sources of Funding: None declared. Ethical approval: The patient provided written consent in the presence of MC and AS, agreeing to publication of the paper.
References
- Medicines Optimisation Group, Clinical Guidance. subcutaneous administration of tranexamic acid, St Christopher’s, London. 2017.
- Gibbs M, Clinical Guideline. Subcutaneous Infusion of Tranexamic Acid, Ashtons Hospital Pharmacy Services, SITA1 (V2.0).
- Howard P. MEMO: subcutaneous Paracetamol, Tranexamic acid, Esomeprazole and Magnesium sulphate, Mount Batten, Isle of Wight. 2019.
- Summary of Product Characteristics. Tranexamic Acid 100mg/ml Solution for Injection. 2021.
- British National Formulary. BNF is only available in the UK. 2021.
- Couto R, Charafeddine C, Sinclair A, et al. Local Infiltration of Tranexamic Acid with Local Anesthetic Reduces Intraoperative Facelift Bleeding: A Preliminary Report, Aesthetic Surgery Journal. 2020; 40: 587-593.
- Zilinsky I, Barazani T, Martinowitz U, et al. Subcutaneous Injection of Tranexamic Acid to Reduce Bleeding during Dermatologic Surgery: A Double-Blind, Placebo-Controlled, Randomized Clinical Trial, Dermatologic Surgery. 2019; 45: 759-767.
- Sagiv O, Rosenfeld E, Zloto O, et al. Subcutaneous tranexamic acid in upper eyelid blepharoplasty: a prospective randomized pilot study, Canadian Journal of Ophthalmology. 2018; 53: 600-604.