
Special Article - Atherosclerosis
Ann Hematol Oncol. 2022; 9(3): 1399.
Dapagliflozin Attenuates Severity of Atherosclerosis through Manufacturing Energy-Crisis to Inhibit Ferroptosis of Macrophage
Li Z1#, Jia J2#, Hao H1, Qiao S1, Chen J1, Li G1, Qi Y1, Sun X1, Kang L1* and Xu B1*
1Department of Cardiology, Nanjing University Medical School Affiliated Nanjing Drum Tower Hospital, PR China
2Department of Medical Laboratory, Nanjing University Medical School Affiliated Nanjing Drum Tower Hospital, PR China
#These authors contributed equally to this article
*Corresponding author: Lina Kang, MD, PhD, Department of Cardiology, Drum Tower Hospital, Medical School of Nanjing University, No. 321 Zhongshan Road, Nanjing, 210008, China
Biao Xu, MD, PhD, Department of Cardiology, Drum Tower Hospital, Medical School of Nanjing University, No. 321 Zhongshan Road, Nanjing, 210008, China
Received: June 24, 2022; Accepted: July 26, 2022; Published: August 02, 2022
Abstract
Background: Atherosclerosis is a main potential pathology of most cardiovascular diseases. Growing evidence has indicated that dysregulation of ferroptosis is associated with atherosclerosis. Dapagliflozinas a sodiumglucose cotransporter 2 inhibitor (SGLT2i), in view of the clinically important benefits of the sodium-glucose cotransporter 2 inhibitor (SGLT2i) dapagliflozin in improving cardiovascular outcomes, we aimed to explore its pharmacological effects and underlying mechanisms of atherosclerosis associated with ferroptosis.
Method: Ferroptosis and severity of atherosclerosis were assessed in ApoE-/-(control) and ApoE-/-+Dapagliflozin (SGLT2i) mice groups to analyze the changes after Dapagliflozin treatment. ApoE-/-+Dapagliflozin+RSL3 (RSL3) mice group was established to confirm the association between ferroptosis and severity of atherosclerosis. Metabolism of nutrients and energetic phenotype of macrophages sorted by FACS within atherosclerosis plaques were analyzed to explore the mechanism of Dapagliflozin attenuating the severity of atherosclerosis.
Result: Alleviated severity of atherosclerosis and ferroptosis were observed in Dapagliflozin treatment mice group, however, RSL3 treatment mice group revoke the beneficial effect. The metabolism of macrophages sorted by Fluorescence Activated Cell Sorter (FACS) with in atherosclerosis plaques indicated that Dapagliflozin treatment weakened fuel flexibility between mitochondrial respiration and nutrients consumption which leads to energy crisis within macrophages. The energy crisis mitigates ferroptosis and keeps more M2 macrophages surviving in atherosclerotic plaques.
Conclusion: Dapagliflozin treatments modulate atherosclerotic plaque progression and maintain plaque stabilization by the way of creating energy crisis. Energy crisis keeps more M2 macrophages surviving in atherosclerotic plaque which is more sensitive to ferroptosis than M1 to attenuate severity of atherosclerosis. The pharmacological effects of Dapagliflozin might be a potential new therapeutic medication target for atherosclerosis lesions.
Keywords: Dapagliflozin; Ferroptosis; Atherosclerosis; Energy crisis; Macrophages
Introduction
Dapagliflozin as a sodium glucose cotransporter 2 inhibitor (SGLT2i) has been already developed as an effective hypoglycemic drug that target SGLT2 which is the main glucose transporter responsible for approximate 90 %glucose reabsorption from primary urine in the kidney [1]. Recently, lots of evidences have suggested Dapagliflozin affects on reducing body weight, glycosylated hemoglobin, plasma volume, blood pressure, increasing erythrocyte mass, and improving cardiac energy metabolism, which imposes many positive influences on the vast majority of cardiovascular risk factors and outcomes [2-4]. In view of the major clinical benefits of Dapagliflozin in improving cardiovascular outcomes, we propose a comprehensive and insightful theory of its pharmacological effects and underlying mechanisms in Cardiovascular Disease (CVD) prevention, which focus on addressing the mechanism of accelerating atherosclerosis associated with ferroptosis.
Ferroptosis is a nonapoptotic form of cell death regulated, which is induced by the over-production of phospholipid hydroperoxides in an iron-dependent manner [5-7]. Dysregulation of ferroptosis is well known to associate with various pathological conditions and major diseases, such as atherosclerosis, ischemia-reperfusion, neurodegeneration and cancer [8]. Whereas, RSL3 is identified as a potent ferroptosis triggering agent, which depends on the activity of GPX4 (Shintoku et al., 2017). GPX4 to mediate suppression of ferroptosis (Friedmann Angeli et al., 2014; Yang et al., 2014).
Atherosclerosis is a main potential pathology of most CVDs, including acute myocardial infarction (AMI), Heart Failure (HF), stroke, and peripheral arterial disease. CVDs are generally acknowledged as the leading cause of morbidity and mortality globally [9]. Atherosclerosis is a slow-progressing inflammatory process which includes a complex biochemical and cellular etiology characterized by the deposition of modified lipids in the arterial walls, the progress of lipid-laden atherosclerotic plaque and ultimate rupture of the plaque which precipitates a lethal clinical event being a heart attack or stroke [10]. The clinically conventional risk factors for atherosclerosis and its complications include hypertension, overweight, smoking, dyslipidemia, depression, sedentary lifestyles and diabetes [11,12]. In particularly, it has relationships between obesity, diabetes and dyslipidemia with dysregulation of ferroptosis. Patients with those conventional risk factors, especially those patients who already confirmed metabolic abnormalities have a higher risk of atherosclerosis and other complications associated with ferroptosis [13-15].
Normal cells require an adequate supply of nutrients and energy to survive and perform function. The deficiencies in nutrients and energy lead to a metabolic crisis [16,17]. One kind of metabolic crisis is called energy crisis, which is characterized by the deficiency of intracellular ATP. Initially, energy crisis leads to adaptive response, which induces reestablish energy homeostasis, however, under longterm and severe energy crisis with excessive deficiency of intracellular ATP, such adaptive responses cannot restore the energy balance and unresolved energy crisis eventually induces apoptosis. Whether the energy crisis regulates other nonapoptotic forms of cell death such as ferroptosis remains largely unknown.
In our study, we identified Dapagliflozin attenuated atherosclerosis by inhibiting ferroptosis of M2 macrophages through leading an energy crisis [18]. So Dapagliflozin might be a potential new therapeutic medication target for atherosclerosis lesions in clinic.
Methods
Animal
All mice were fed with a standard laboratory diet and free accessed to food and water. Mice were kept in a temperature (20-22°C) and humidity (65%-70%) controlled room, with a 12-h light-dark cycle. All mice were anesthetized by isoflurane inhalation (1.5%-2%) and then euthanized by cervical dislocation at the end of experiments. Female mice were not used in the study due to the possible effect of estrogen, such as estrogen and menstrual periods affecting food in taking which may affect the metabolism. ApoE-/- were all purchased from the Shanghai Model Organisms Center.
Establishing Mouse Model
Atherosclerotic lesions models were established on 6-week male ApoE-/- mice and mice were randomly grouped into the ApoE-/-(control), ApoE-/-+Dapagliflozin (SGLT2i) and ApoE-/- +Dapagliflozin+RSL3 (RSL3) mice groups then fed with a high-fat and cholesterol diet (Research DIETS Co., Ltd., USA) for more than 3 months. For Dapagliflozin treatment group, 0.25mg Dapagliflozin dissolved in 0.1mL 0.9% NS (room temperature keep vibration till suspension) then treated ApoE-/- mice through intragastric administration once a day and for RSL3 treatment group, except the treatment of Dapagliflozin, 2.5mg RSL3 dissolved in 0.1 mL CMCNa- NS (0.5g CMC-Na dissolved in 100mL 0.9% NS room temperature overnight and ultrasonic heating at 50-60°C for 30 minutes) and then treated ApoE-/- mice through intragastric administration twice a week.
Pathological Analysis Protocol
The aortas tissues isolated from ApoE-/-(control), ApoE-/- +Dapagliflozin (SGLT2i) and ApoE-/-+Dapagliflozin+RSL3 (RSL3) mice groups were measured. Hematoxylin & Eosin (HE) staining was used to assess inflammation. The aorta tissues were put in 10% formaldehyde solution, followed by dehydration in an ethanol gradient. Paraffin embedded tissues were cut down into slices of 4μm. After deparaffinized, the samples were stained with hematoxylin and eosin, mounted and observed under a light microscope (Leica Microsystems, Wetzlar, Germany).Masson and a-SMA staining have a similar process. En face aorta oil red O staining was used to assess the severity of atherosclerosis. The aorta tissues were excised, andthen embedded in an optimal cutting temperature, compound, frozen on dry ice, and then stored at -80°C until sectioning for Hematoxylin and Eosin (H&E), oil red O.
TEM
TEM analysis was performed by High-Resolution Electron Microscopy. Samples were fixed with a solution containing 3% glutaraldehyde plus 2% paraformaldehyde in 0.1ml cacodylate buffer, pH7.3, then washed in 0.1ml sodium cacodylate buffer and treated with 0.1% Millipore-filtered cacodylate-buffered tannic acid, post fixed with 1% buffered osmium and stained with 1% Milliporefiltered uranyl acetate. The samples were dehydrated in increasing concentrations of ethanol, infiltrated and embedded in an electron microscope fixative. The samples were polymerized in a 60°C oven for approximate 3days. Ultrathin sections were cut in a Leica Ultracut microtome (Leica), stained with uranyl acetate and lead citrate in a Leica EM Stainer and examined in a JEM 1010 transmission electron microscope (JEOL, USA, Inc.) at an accelerating voltage of 80 kV. Digital images were obtained using the AMT Imaging System (Advanced Microscopy Techniques Corp.)
Seahorse XF
Analysis of Oxygen Consumption Rate (OCR) and Extracellular Acidification Rate (ECAR), indicators of mitochondrial aerobic respiration and glycolytic activity, respectively, were measured using the Seahorse XFe96 system (Agilent Technologies, Santa Clara, CA). Cells were seeded at 2×106 cells per well. Cells were attached to the bottom of the wells using 3.5 mg/cm² CellTak (Corning, Corning, NY) allowing data normalization by cell number. The XF Cartridge was hydrated in ddH2O overnight at 37°C in non-CO2 containing incubator. Cells and treatments were done in Seahorse media supplemented similarly to cell media depending on assay performed (2g/l GLU,1 mM sodium pyruvate, 2mM L-glutamine). The cell energy phenotype test resulted from a concentration of 3mM FCCP (mitochondrial membrane depolarizer)/1mM Oligomycin (OM) (ATP-synthase inhibitor). Data are presented as percent control, with control set at 100%.
Cell Mitochondrial Stress Test
The cell mitochondrial stress test resulted from a concentration of 1 mM OM, concentration 3mM FCCP, and a concentration mixture of 0.5mM rotenone (complex 1 inhibitor) and 0.5mM antimycin A (complex 3inhibitor) (ROT/AA).
Cell Glycolysis Stress Test
The glycolysis stress test resulted in a concentration 10 mM GLU, concentration 1 mM OM, and concentration 50 mM 2-deoxyglucose (2-DG) (hexokinase inhibitor) (2-DG).Media for this test did not contain GLU or sodium pyruvate.
Mitochondrial Fuel Flexibility Stress Test
The mitochondrial fuel flexibility stress test used sequential injection of metabolic fuel pathway inhibitors to test fuel flexibility. Final well concentration of inhibitors was 2 mMUK5099 (inhibitor of the GLU oxidation pathway), 4mM etomoxir (inhibitor of long chain fatty acid oxidation), and 3 mM BPTES (inhibitor of glutamine oxidation). For each nutrient, the dependence was calculated by dosing cells with inhibitor of nutrient of interest in port B followed by mixture of other two inhibitors in port C. This will demonstrate the cell’s reliance on specific fuel source to maintain respiration. Capacity was calculated by dosing inhibitors in reverse: the inhibit oxidation of two nutrients in port B and then inhibit nutrient of interest in port C. This demonstrates ability of cell to up regulate one specific fuel source when the other two are inhibited. Fuel flexibility is the difference of dependence and capacity and describes the cell’s ability to increase the oxidation of a specific nutrient source to compensate for inhibition of the other two.
Statistics Analysis
Data from at least five independent experiments were presented as mean ± SD, unless indicated. To determine differences between groups at a single time point, data were tested using either 2-tailed, unpaired, Student’s t-test or 1-way ANOVA followed by Tukey’s multiple comparisons test. All analyses were performed using Prism 6 software (GraphPad), and only differences with a P value of less than 0.05 were considered statistically significant.
Results
Dapagliflozin Treatment Attenuates Severity of Atherosclerosis
To examine the effect of Dapagliflozin on atherosclerosis, we divided mice into groups of ApoE-/-(Control), ApoE-/-+Dapagliflozin (SGLT2i) and ApoE-/-+Dapagliflozin+RSL3 (RSL3) (Figure 1A) then fed with a high-fat and cholesterol diet (HFD, Research DIETS Co., Ltd., USA) for more than 3 months. The analysis of atherosclerosis lesions revealed that the plaque areas (Figure 1B) and plaque vulnerability in Dapagliflozin mice group were reduced compared with the control mice group, meanwhile, the inflammatory cell infiltration reduced within atherosclerotic plaque (Figure 1C). Our results confirmed the efficacy of artery protection of Dapagliflozin.
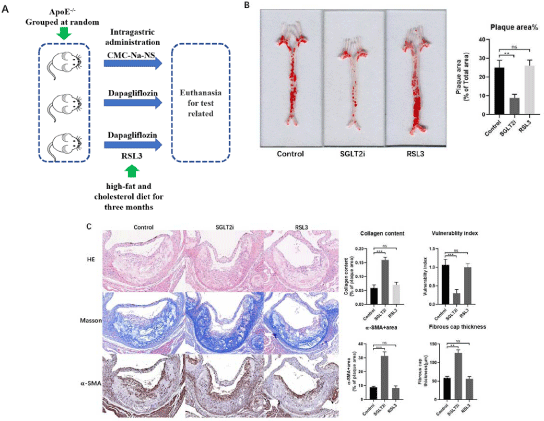
Figure 1: (A) Atherosclerosis lesions within arteries wall in control, Dapagliflozin and RSL3 mice groups. Using oil red O staining on the whole aorta to measure
quantification of artery lesion area evaluating vulnerability of atherosclerosis lesions. Scale bar = 400μm. (B) Evaluating the vulnerability of atherosclerosis lesions,
HE, Masson and a-SMA staining. Scale bar = 400μm.
Dapagliflozin Inhibits Ferroptosis in Atherosclerosis
As dysregulation of ferroptosis is associated with various pathological conditions of human diseases, for instance, atherosclerosis, ischemia-reperfusion, neurodegeneration and cancer. For clarity underlying mechanisms of Dapagliflozin in CVD prevention, we take the atherosclerosis lesions for the analysis of redox reactions referring to ferroptosis. Our results revealed that the index of antioxidant system, GSH, GSH/GSSG increased at a large amount in Dapagliflozin mice group, which inhibited ferroptosis and protected mitochondria in atherosclerosis (Figure 2A) (Figure 2B). These results show Dapagliflozin inhibits ferroptosis in atherosclerosis.
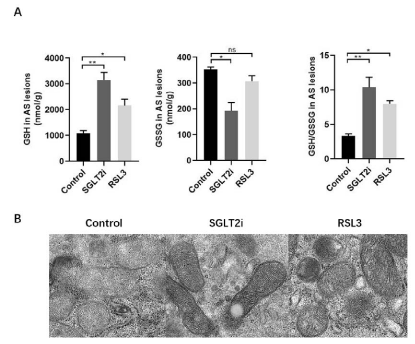
Figure 2: (A) Analysis of ferroptosis within the arteries wall in control, Dapagliflozin and RSL3 mice groups. (B) Ferroptosis of mitochondria within the arteries wall
in control, Dapagliflozin and RSL3 mice groups.
Dapagliflozin Treatment Attenuates the Severity of Atherosclerosis through Inhibiting Ferroptosis
To confirm our hypothesis of Dapagliflozin treatment attenuated the severity of atherosclerosis through inhibiting ferroptosis, RSL3,which can induce ferroptosis was dissolved in CMC-Na-NS to treat Dapagliflozin pretreated mice group through intragastric administration twice a week for another 3 months (RSL3 mice group). After analysis of the atherosclerosis lesions, the results revealed that compared with only Dapagliflozin treatment mice group, the effect of Dapagliflozin to attenuate the severity of atherosclerosis was revoked inRSL3 mice group (Figure 1 & 2).
Dapagliflozin Inhibits-ferroptosis of M2 Macrophages within Atherosclerotic Plaques
As known, macrophages play a core role in atherosclerosis progression and different subtypes of macrophages have been detected within atherosclerotic plaques. M1 macrophagesare pro-atherogenic and considered plaque-promoting and destabilizing atherogenic. On the other hand, M2 macrophages are thought to counterbalance inflammatory responses, promoting stabilization of plaques [19,20]. Moreover, iron turnover is different in M1 and M2 cells. Due to the low expression of FPN and Haemoxygenase-1(HO-1), M1 macrophages are more tolerated of ferroptosis than M2 macrophages [21,22]. Therefore, we analyzed the ferroptosis of macrophages and M2 macrophages survived within the atherosclerotic plaques in control and Dapagliflozin mice groups. Our results released that the index of antioxidant system, GSH/GSSG increased with in monocyte macrophage (F4/80+&CD11b+) sorted by FACS from Dapagliflozin mice group (Figure 3A), meanwhile, more M2 macrophages (CD206+) survived within plaques in Dapagliflozin mice group compared with control mice group (Figure 3B).
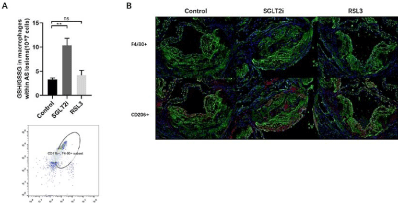
Figure 3: (A) Analysis of ferroptosis of macrophages within the arteries wall in control, Dapagliflozin and RSL3 mice groups. (B) M2 macrophages within the arteries
wall in control, Dapagliflozin and RSL3 mice groups.
Dapagliflozin Mediates Energy Crisis to Inhibits- Ferroptosis of M2 Macrophages
Dapagliflozin is the inhibitor of the major glucose transporter in the kidney responsible for approximate 90% of glucose reabsorption from primary urine, which causes energy crisis led by glucose starvation. As Hyemin Lee etc. reporting that energy crisis inhibits ferroptosis, we furtherly hypothesized whether Dapagliflozin inhibits ferroptosis of M2 macrophages led to the energy crisis [23,25]. We analyzed energy metabolism of macrophages sorted by FACS using Seahorse XF fuel flexibility stress test through combinations of inhibitors that block metabolism of nutrients necessary for mitochondrial respiration (Glucose, Long chain fatty acids and Glutamine) [26-31]. By dosing cells with different combinations of nutrient pathway inhibitors after treatments, the results came out that the ability of M2 macrophage to switch between fuel sources was compromised after Dapagliflozin treatments. The Dependence of M2 macrophage on Glucose was significantly increased after Dapagliflozin treatments. At the same time, the capacity of M2 macrophage to up regulate Glucose oxidation was showed no significantly changes. Next, the Dependence of M2 macrophage on Fatty acid oxidation after Dapagliflozin treatments was increased and the capacity to upregulate Fatty acid oxidation was also showed no changes. The last nutrient we tested was Glutamine. M2 macrophage after Dapagliflozin treatments led to a trend of higher dependence while still no significant change of Glutamine oxidation in capacity after Dapagliflozin treatments (Figure 4A). By combining dependence and capacity, we calculate that there was a consistent pattern of decreased fuel flexibility in M2 macrophage with Dapagliflozin treatments across all three nutrients compared with the other two mice groups. The control and RSL3 mice groups showed the similar tendency (Figure 4B).
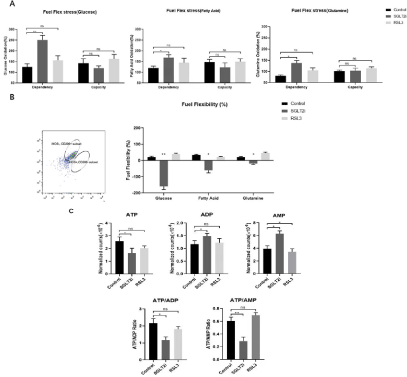
Figure 4: (A) Analyze energy metabolism of macrophages sorted by FACS using Seahorse XF fuel flexibility stress test (nutrients of Glucose, Long chain fatty acids
and Glutamine). (B) Fuel flexibility in M2 macrophage within arteries wall in control, Dapagliflozin and RSL3 mice groups. (C) Ratios of ATP/ADP and ATP/AMP.
We next explored metabolite profile of molecules directly reflecting energy production and use in the purinergic pathway. As known, calculating the ratios of ATP/ADP and ATP/AMP provides a quantification of the cellular shift toward energetic phenotype. In our results, both ATP/ADP, ATP/AMP ratios and ATP trended toward a significant decrease in M2 macrophage with Dapagliflozin treatments which indicated a cell energy crisis (Figure 4C). Our results indicated that Dapagliflozin treatments eliminates fuel flexibility between mitochondrial respiration nutrients which leads an energy crisis within monocyte macrophage. The energy crisis relieves ferroptosis and keeps more monocyte macrophage polarized to M2 macrophages when they are suffered from ferroptosis. More M2 macrophages survived within atherosclerotic plaque, so the severity of atherosclerosis was attenuated and its stabilization was maintained.
Discussion
Glucose is the most principal nutrient required in most cells to maintain biosynthetic, bioenergetics and redox homeostasis to maintain sufficient energy. As ferroptosis is induced by lipid peroxidation, a type of ROS-mediated lipid damage which consumes energy, it seems logical that, glucose starvation should mild ferroptosis because of energy crisis. In our study, we confirmed our hypothesis that the energy crisis led by glucose starvation largely prevents M2 macrophages from mitigating ferroptosis, meanwhile, we found that Dapagliflozin treatments induce or mimic certain type of energy crisis to inhibit ferroptosis within atherosclerotic plaques. Previous research has already reported the benefits of Dapagliflozin treatments attenuate the severity of atherosclerosis and M2 macrophages were confirmed to maintain stabilization and reduce inflammation in previous studies [15,20]. Above all, we came with the conclusion that Dapagliflozin treatments attenuate the severity of atherosclerosis through inhibiting ferroptosis mediated by energy crisis. So far there is no mechanism studies related to energy crisis and ferroptosis. So, our research maybe the first time to reveal their correlations. However, there were no human specimen of atherosclerotic plaque in our studies and basically, we should start from clinical data.
Conclusion
Our current data show that energy crisis led by Dapagliflozin treatments prevents M2 macrophages from mitigating ferroptosisto attenuate the severity of atherosclerosis. Therefore, Dapagliflozin may be an effective treatment for counterbalance inflammatory responses, reducing plaque formation and promoting plaque stabilization.
Ethics Approval and Consent to Participate
All animal experiments were approved by the Institutional Ethics Committee of Nanjing University Medical School Affiliated Nanjing Drum Tower Hospital (Approval No. 20011141) and followed the guidelines set forth in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Eighth Edition).
Consent for Publication
I would like to declare on behalf of all my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
No conflict of interest exited in the submission of this manuscript, and the manuscript is approved by all authors for publication.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (grant number 82070366), and the Key Project of Science and Technology Development of Nanjing Medicine (grant number ZKX20018).
Authors Contributions
Designed the study: Zhu Li, Lina Kang, Biao Xu
Performed the research: Zhu Li, Jia Jia, Han Hao, Shiyang Qiao
Analyzed data: Zhu Li, Jia Jia
Wrote the paper: Zhu Li
Assist work: Jianzhou Chen, Guannan Li, Yu Qi, Xuan Sun
Acknowledgments
The authors thank Jinkui Xue and Rui Deng for their technical expertise and guidance.
References
- Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. The Journal of clinical investigation. 2014; 124: 499-508.
- Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: Proposed pathways and review of ongoing outcome trials. Diabetes & Vascular Disease Research. 2015; 12: 90-100.
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. The New England journal of medicine. 2015; 373: 2117-2128.
- Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, et al. Rationale, design, and baseline characteristics of a randomized, placebocontrolled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME™). Cardiovascular Diabetology. 2014; 13: 102.
- Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell. 2012; 149: 1060-1072.
- Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cellular and Molecular Life Sciences. 2016; 73: 2195-2209.
- Yang WS, Stockwell BR. Ferroptosis: Death by Lipid Peroxidation. Trends in cell biology. 2016; 26: 165-176.
- Mjiyad NE, Caro-Maldonado A, Ramírez-Peinado S, Muñoz-Pinedo C. Sugar-free approaches to cancer cell killing. Oncogene. 2011; 30: 253-264.
- Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE-/- mice fed a western diet. Diabetologia. 2016; 60: 364-376.
- Koh KK. Letter by Koh Regarding Article, “Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhi. Circulation. 2018; 137: 984-985.
- Tahara A, Takasu T, Yokono M, Imamura M, Kurosaki E. Characterization and comparison of sodium-glucose cotransporter 2 inhibitors: Part 2. Antidiabetic effects in type 2 diabetic mice. Journal of pharmacological sciences. 2016; 131: 198-208.
- Lim VG, Bell RM, Arjun S, Kolatsi-Joannou M, Long DA, Yellon DM. SGLT2 Inhibitor, Canagliflozin, Attenuates Myocardial Infarction in the Diabetic and Nondiabetic Heart. JACC: Basic to Translational Science. 2019; 4: 15-26.
- Leng W, Ouyang X, Lei X, Wu M, Chen L, Wu Q, et al. The SGLT-2 Inhibitor Dapagliflozin Has a Therapeutic Effect on Atherosclerosis in Diabetic ApoE-/- Mice. Mediators of Inflammation. 2016; 2016: 1-13.
- Díaz-Rodríguez E, Agra RM, Fernández L, Adrio B, García-Caballero T, González-Juanatey JR, et al. Effects of dapagliflozin on human epicardial adipose tissue: modulation of insulin resistance, inflammatory chemokine production, and differentiation ability. Cardiovascular Research. 2018; 114: 336-346.
- Shigiyama F, Kumashiro N, Miyagi M, Ikehara K, Kanda E, Uchino H, et al. Effectiveness of dapagliflozin on vascular endothelial function and glycemic control in patients with early-stage type 2 diabetes mellitus: DEFENCE study. Cardiovascular Diabetology. 2017; 16.
- Sugiyama S, Jinnouchi H, Kurinami N, Hieshima K, Yoshida A, Jinnouchi K, et al. The SGLT2 Inhibitor Dapagliflozin Significantly Improves the Peripheral Microvascular Endothelial Function in Patients with Uncontrolled Type 2 Diabetes Mellitus. Internal Medicine. 2018; 57: 2147-2156.
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012; 13: 251-262.
- Stockwell BR, Angeli JPF, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017; 171: 273-285.
- Xu L, Ota T. Emerging roles of SGLT2 inhibitors in obesity and insulin resistance: Focus on fat browning and macrophage polarization. Adipocyte. 2018; 7: 1-8.
- Xu L, Nagata N, Nagashimada M, Zhuge F, Ni Y, Chen G, et al. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine. 2017; 20: 137-149.
- Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, et al. Global Survey of Cell Death Mechanisms Reveals Metabolic Regulation of Ferroptosis. Nature chemical biology. 2016; 12: 497-503.
- Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh HJ, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016; 12: 1425-1428.
- Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and Transferrin Regulate Ferroptosis. Molecular cell. 2015; 59: 298-308.
- Koppula P, Zhang Y, Zhuang L, Gan B. Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Communications. 2018; 38: 12.
- Bianchi A, Evans JL, Nordlund AC, Watts TD, Witters LA. Acetyl-CoA carboxylase in reuber hepatoma cells: Variation in enzyme activity, insulin regulation, and cellular lipid content. Journal of Cellular Biochemistry. 1992; 48: 86-97.
- Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Research. 2016; 26: 1021-1032.
- Kagan VE, Mao G, Qu F, Angeli JPF, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nature chemical biology. 2017; 13: 81-90.
- Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nature chemical biology. 2017; 13: 91-98.
- Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of Mitochondria in Ferroptosis. Molecular cell. 2019; 73: 354-363.
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nature Reviews Molecular Cell Biology. 2018; 19: 121-135.
- Jeon S, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012; 485: 661- 665.