
Original Research
Ann Hematol Oncol. 2022; 9(4): 1403.
Common Genetic Variants of Fetal Hemoglobin Modify Hematological Phenotypes in MDS/MPN/Myeloma Patients Receiving Cytotoxic Drugs
Igbineweka NE1,2,3,4, Pilarski A1,2, Rooks H3, Breton A3, Hall H1, Joshi D1, Meeks D1,2,4, Chevassut TJ1,2, Thein SL4 and Menzel S3*
1Brighton & Sussex Medical School, University of Sussex, UK
2Department of Haematology, Brighton & Sussex University Hospitals NHS Trust, UK
3Molecular Haematology, School of Cancer and Pharmaceutical Sciences, The Rayne Institute, King’s College London, UK
4Sickle Cell Branch, National Heart, Lung and Blood Institute, National Institute of Health, USA
*Corresponding author: Stephan Menzel, School of Cancer and Pharmaceutical Sciences, The Rayne Institute King’s College London, 123 Coldharbour Lane, London SE5 9NU, UK
Received: August 26, 2022; Accepted: September 15, 2022; Published: September 22, 2022
Abstract
Genetic studies identify common variants within the HBS1L-MYB intergenic region (HMIP), BCL11A, and Xmn1-HBG2 as associated with elevated fetal hemoglobin (HbF) levels and other clinically important human hematological traits. Recent studies suggest HbF is a predictor of outcome in MDS/AML patients receiving decitabine. We assessed effects of HbF genetic variants on hematological traits in Myeloproliferative Neoplasm (MPN), Myelodysplastic Syndrome (MDS) and myeloma on HbF-inducing therapy to determine potential for variants predicting treatment response. Seven common HbF variants at HMIP, BCL11A, Xmn1-HGB2 loci were genotyped in 89 patients with MPN on Hydroxyurea (HU), myeloma on Lenalidomide, and MDS on Azacytidine. HbF genetic association was seen with rs9494142 (HMIP) in MPN on HU (p = 0.04) and rs1427407 (BCL11A) in myeloma on Lenalidomide (p = 0.002). HMIP variants rs9494142 and rs6920211 influenced baseline platelets (p = 0.04) and hemoglobin treatment response (p = 0.02). rs1427407 (BCL11A) was significantly associated with increased platelets (p = 0.04) negating thrombocytopenic tendency of Lenalidomide. These HbF variants showed significantly discordant minor allele frequencies in MDS/MPN/myeloma compared to wider European population data. This small study finding together suggest the implication of these variants in treatment response and disease biology in MDS/MPN/myeloma warranting larger prospective genotype-phenotype association studies.
Keypoints
• HbF genetic variants influence HbF and other hematological traits in MPN, myeloma and MDS patients at baseline and response to HbF-inducing cytotoxic therapy.
• Frequencies of minor alleles of HBS1L-MYB and BCL11A are significantly discordant compared with wider population genomic data suggesting a pathobiological role of these loci in MPN, myeloma and MDS.
Introduction
Fetal hemoglobin, although the predominant hemoglobin until birth, continues to be synthesized in minimal amounts throughout adult life. [1] Some reactivation of fetal hemoglobin (HbF, alpha2 gamma2) in adults can occur in certain hematopoietic states such as Myelodysplastic Syndrome (MDS), Myeloproliferative Disorders (MDS), and Sickle Cell Disease (SCD); sometimes as a response to therapy, but occasionally prior to any intervention. [2] The significance of elevated HbF levels for patients towards the understanding of disease mechanisms is so far unclear. It is conceivable that HbF could be a marker for imbalanced or clonal hematopoiesis, or, alternatively, for committed pathways of the hematopoietic system to cope with disease. It could be a sign of effective therapeutic intervention or represent stress inflicted on the bone marrow by therapy. [1] In some cases, higher HbF levels could have been present before the onset of disease, but the causes of this could have influenced risk for the development of manifest disease, or impacted disease progression. [4,5] While HbF can just be a reaction to disease or therapy, there is also the possibility that hematopoietic processes leading to increased HbF are part of the pathogenetic process itself, influencing disease risk, progression or treatment efficacy. It appears beneficial to dissect these possible relationships between increased HbF levels and certain hematopoetic disease states, since HbF levels could indeed have diagnostic or prognostic value, or understanding its causes could help to understand disease itself. Lübbert et al. [4] suggested recently that baseline HbF had prognostic value in predicting outcome in MDS/ AML patients receiving decitabine, but genetic variants associated with HbF were not evaluated which could have provided insight for genetic prediction of treatment response and prognosis.
HbF has high heritability, and like other hematological traits, is tightly genetically regulated. [6-9] HbF genetic regulation has been well delineated in hemoglobinopathies and inherited bone marrow failure syndromes. [10] Three Quantitative Trait Loci (QTL) accounts for 20-50% of HbF common variation - HBS1L-MYB intergenic polymorphism (HMIP) on chromosome 6q11, BCL11A on chromosome 2p [12] and Xmn1-HBG2 (rs7482144) on the β-globin cluster on chromosome 11p [13]. HbF variants such as HMIP and BCL11A have also been shown to regulate other hematological traits. [14-16] In addition, BCL11A and Xmn1-HBG2 (rs7482144) has been suggested to influence HbF response to hydroxyurea (HU) in the β-hemoglobinopathies. [17] Nevertheless, there remains a paucity of data describing significance of HbF variants amongst hematological malignancies.
This study investigates HbF regulatory potential variant modifier effects and prevalence amongst patients with MPN/myeloma/MDS on cytotoxic therapy; agents which are also used for HbF therapeutic reactivation in SCD and beta-thalassemia. [18-21] HbF response to these chemotherapy agents are likely to be highly variable and genetically influenced reflecting alterations of hematological kinetics and improvement of blood counts. Delineation of these HbF variants, could allow for added insight to disease risk and expression, molecular characterization, and prediction of response to these chemotherapy agents in MPN/Myeloma/MDS.
Methodology
Subject group
Eighty-nine patients, 44 with MPN (World Health Organization (WHO) classification 2008) on HU; 33 patients with Myeloma (International Myeloma Working Group 2003) on Lenalidomide; and 12 patients with MDS (WHO classification 2001) on Azacytidine (AZA) were recruited from hematology clinics at Brighton & Sussex University Hospitals (BSUH) NHS Trust, UK (supplemental data (S) Figure S1). Four out of the 12 patients with MDS, had secondary AML at the time of measurement. A subset of patients (N = 16) (7 MPN, 6 myeloma and 3 MDS patients) had baseline values before starting therapy and had repeat values 4 weeks and 3-months after cytotoxic agent initiation. All patients gave written consent. The study was approved by local and national UK NHS Research Ethics Committee (REC) (reference 14/WA/1078).
Blood Sampling & Genotyping
Two EDTA blood samples were collected from each patient, one processed at BSUH pathology for HbF using HPLC (BioRad variant) and the other processed for hematological parameters (Sysmex XN- 9000 automated hematology analyzers) (Norderstedt, Germany). Elevated HbF was defined as ≥ 1%.
DNA was extracted from peripheral blood leukocytes from the second EDTA sample and genotyped for 7 HbF variants - rs6545816 and rs1427407 (BCL11A), rs9494142, rs6920211, rs9494145, and rs66650371 (HMIP), and rs748214 using Taqman procedure (standard assay system and MGB probes from Thermo Fisher/ Applied Biosystems, Foster City, CA).
Statistical Analysis
Genetic association of blood traits was performed with linear regression (IBM SPSS Statistics 24 & GraphPad software, La Jolla, CA, USA) using a genotypic genetic model adjusting for age and sex as covariates due to HbF levels being lower with increasing age and higher levels observed in females compared to males. Analysis of variance was performed to determine genotype effect against mean HbF/hematological parameters. Hardy-Weinberg test was performed on all genotype results for quality assurance. No genotype was found to deviate from the Hardy-Weinberg equilibrium. Hematological parameters which did not satisfy assumptions of normality were logtransformed. Statistical significance was determined as a p-value of < 0.05 with Bonferroni-adjustment for multiple testing.
Results
Fetal Hemoglobin Is Variably Expressed In MPN/Myeloma/ MDS at Baseline (Pre-Cytotoxic Treatment) and On Induction (Post-Cytotoxic Treatment)
We studied general blood parameters, HbF levels, and genetic HbF modifiers in 89 patients with hematological malignancy (MPN, Myeloma and MDS, Table 1) in relation to HbF-inducing cytotoxic therapy and genetic modifiers of HbF expression. In 16 of the 84 patients – 7 MPN, 6 myeloma and 3 MDS – we were able to compare baseline values before starting therapy with values obtained 4 weeks and 3 months after cytotoxic agent initiation. Mean baseline HbF for this total group was 0.78% (Table 1) with 0.73%, 0.70%, 0.95% for MPN, Myeloma and MDS respectively (Table S1). Four patients had elevated baseline HbF (2 MPN, 1 Myeloma and 1 MDS) with maximal elevated baseline HbF level of 1.2% seen in two patients (1 MPN, 1 MDS). Mean induced HbF levels were 1.06%, 0.77%, and 2.36% in MPN on HU, Myeloma on Lenalidomide and MDS on AZA groups respectively. Mean induced HbF levels for the total hematological malignancy group (MPN/myeloma/MDS) was 1.08%. Elevated HbF was prevalent amongst 28.6% MPN on HU, 19% Myeloma on Lenalidomide and 63.6% MDS on AZA groups.
General characteristics
Total hematological malignancies
Myeloproliferative neoplasm
Myeloma
Myelodysplastic Syndrome
Baseline (pre- treatment) cohort
(n = 89)
(n = 44)
(n = 33)
(n = 12)
(n = 16)
Age (years) (mean ± SD)
73.7 ± 10.8
74 ± 10.73
74 ± 9.4
72.7 ± 5.2
70.3 ± 11.3
Age range (years)
47 - 96
49 - 88
44 - 96
47 - 83
47 - 83
Ethnicity (White British) (100%)
Sex, n (%)
Male
50 (56.2)
23 (52.3)
17 (51.5)
10 (83.3)
9 (56.2)
Female
39 (43.8)
21 (47.7)
16 (48.5)
2 (16.7)
7 (43.8)
Disease sub-group, n (%)
Myeloproliferative Neoplasm:
44 (49.4)
NA
NA
NA
7 (43.8)
a) Polycythemia Vera
26 (29.2)
26 (59.1)
NA
NA
4 (25)
b) Essential thrombocythemia
16 (18)
16 (36.4)
NA
NA
3 (18.8)
c) Myelofibrosis
2 (2.2)
2 (4.5)
NA
NA
0
Myeloma
33 (37.1)
NA
NA
NA
6 (37.5)
Myelodysplastic Syndrome:
12 (13.5)
NA
NA
NA
3 (18.8)
a) Refractory anemia with excess blasts 2 (RAEB – 2)
4 (4.5)
NA
NA
4 (33.3)
1 (6.3)
b) Refractory anemia with excess blasts in transformation (RAEB – t)
1 (1.1)
NA
NA
1 (8.3)
0
c) Unspecified
3 (3.4)
NA
NA
3 (25)
2 (12.5)
d) Acute Myeloid Leukemia transformation
4 (4.5)
NA
NA
4 (33.3)
0
Cytotoxic agent, n (%)
87 (97.8)
42 (95.5)
32 (96.7)
11 (91.7)
13 (81.3)
a) Hydroxyurea
42 (47.2)
42 (95.5)
NA
NA
4 (25)
b) Lenalidomide
32 (36)
NA
32 (96.7)
NA
6 (37.5)
c) Azacitidine
11 (12.4)
NA
NA
11 (91.7)
3 (18.8)
d) Anagrelide
2 (2.2)
2 (4.5)
NA
0
0
Not on cytotoxic agent, n (%)
2 (2.2)
0(0)
1 (3.03)
1 (8.3)
3 (18.8)
a) Venesection
2 (2.2)
2 (4.5)
0
0
0
Median duration on cytotoxic (days)
194
320.5
199.5
1015
NA
Duration on cytotoxic range (days)
37 - 4169
19 - 4169
37 - 1270
201 - 1158
NA
Hematologic parameters:
Fetal Hemoglobin HbF % (mean ± SD)
1.08 ± 1.41
1.06 ± 1.4
0.77 ± 0.54
2.36 ± 2.5
0.78 ± 0.40
HbF % range
0.2 - 9.1
0.2 - 8.6
0.2 – 2.6
0.5 – 9.1
0.3 - 1.2
n % with elevated fetal hemoglobin (= 1%)
31.5
28.6
19
63.6
4 (25)
Hemoglobin, Hb, g/L (males) (mean ± SD)
116.3 ± 22.3
121.8 ± 23.2
112.8 ± 20.1
109.8 ± 23.2
119 ± 21.2
Hemoglobin, Hb, g/L (females) (mean ± SD)
120 ± 17.6
129.2 ± 12.4
120.5 ± 20.9
90 ± 0
118.3 ± 25.1
n % with anemia (< 135 g/L males; < 115 g/L females)
68.5
47.6
68.8
81.8
9 (56.3)
n % with polycythemia (Hb > 185 g/L males; > 165 g/L females)
0
0
0
0
0
Mean Corpuscular Volume, MCV, f l (mean ± SD)
97.33 ± 13.47
97.3 ± 17.28
97.8 ± 6.1
101.3 ± 10.2
92.5 ± 10.1
n % with macrocytosis (> 100 FL)
42.7
50
34.4
54.5
3 (18.8)
n % with microcytosis (< 76 FL)
7.9
14.3
0
0
1 (6.3)
Red Blood Corpuscles, RBC (mean ± SD)
3.93 ± 1.06
4.3 ± 1.2
3.54 ± 0.55
3.29 ± 0.70
4.1 ± 1.08
Mean Corpuscular Hemoglobin, MCH, pg (mean ± SD)
30.85 ± 6.40
30.3 ± 8.2
31.88 ± 2.2
32.5 ± 3.9
29.9 ± 4.2
White Blood Cell, WBC, 109 / L (mean ± SD)
6.8 ± 4.0
8.7 ± 3.7
4.89 ± 1.84
2.81 ± 1.2
6.5 ± 3.8
n % with leukopenia (< 4 x 109 / L)
18
0
25
54.5
4 (25)
n % with leukocytosis (> 11 x 109 / L)
13.5
23.8
0
9.1
1 (6.3)
Neutrophils, 109 / L (mean ± SD)
4.42 ± 3.53
4.1 ± 3.59
2.59 ± 1.4
1.8 ± 1.55
3.8 ± 3.0
n % with neutropenia (< 2 x 109 / L)
22.5
2.4
40.6
45.5
25
n % with neutrophilia (> 7.5 x 109 / L)
13.5
23.8
3.1
9.1
6.3
Platelets, 109 / L (mean ± SD)
282.5 ± 161.2
378.8 ± 157.7
198.7 ± 112.2
150.2 ± 56
317.9 ± 277.5
n % with thrombocytopenia (< 150 x 109 / L)
20.2
4.8
28.1
54.5
31.3
n % with thrombocytosis (> 450 x 109 / L)
12.4
23.8
3.1
0
4 (25)
Lymphocytes, 109 / L (mean ± SD)
1.53 ± 0.73
1.7 ± 0.67
1.2 ± 0.64
1.2 ± 0.50
1.4 ± 0.67
n % with lymphopenia (< 1.3 x 109 / L)
42.7
26.2
62.5
63.6
31.3
n % with lymphocytosis (> 3.5 x 109 / L)
0
0
0
0
0
Reticulocytes, 109 / L (mean ± SD)
62.13 ± 25.8
69.6 ± 25.9
185.4 ± 123.5
50 ± 20.5
72.4 ± 34.6
n % with reticulocytopenia (< 50 x 109 / L)
28.1
9.5
43.8
45.4
12.5
n % with reticulocytosis (> 100 x 109 / L)
4.5
7.1
3.1
0
12.5
Hematocrit, L/L, (mean ± SD)
NA
0.40 ± 0.05
NA
NA
NA
Lactate Dehydrogenase, LDH, U/L
432.7 ± 243
466.2 ± 295.1
358.8 ± 104.9
437.8 ± 169.7
518.1 ± 258.7
n % with elevated LDH (> 480 U/L)
22.5
26.2
12.5
27.3
43.8
Total Bilirubin, μmol/L
8.22 ± 3.6
8.39 ± 3.13
7.29 ± 3.58
9.8 ± 3.26
9.9 ± 5.5
n % with elevated total bilirubin ( > 22 μmol/L)
0
0
0
0
0
Patients with hematological malignancy in study (n = 89). Table represents data whilst on HbF-inducing cytotoxic agents with exception of 'baseline cohort' which represent pre-treatment data. Doses of cytotoxic agents not provided.
Abbreviations: NA = not applicable;SD = standard deviation; HbF = fetal hemoglobin. HbF levels adjusted for age and sex at baseline.
Table 1: Laboratory values at hospital admission.
HbF Genetic Variants Are Associated with both Baseline and Induced HbF, and Hematological Traits in MPN/ Myeloma/MDS on HbF-Inducing Cytotoxic Therapies
Amongst the total hematological malignancy group, HMIP variants rs9494142 and rs66650371 were statistically associated with higher baseline and induced HbF. rs66650371 associated with significantly lowered White Blood Count (WBC) (p = 0.009) (Table 2). Both HMIP variants rs9494142 and rs66650371 were associated with 175% increase in baseline mean HbF between the homozygous wild and mutant types (p = 0.03) (Table S2). HMIP variant rs6920211 was significantly associated with induced HbF (p = 0.01) amongst this group with added influence reducing WBC (p = 0.03) and small increasing effect on bilirubin (BILI) (p = 0.04). HMIP variants rs6920211 (p = 0.03) and rs66650371 (p = 0.008) were both associated with 43.9% decrease in mean WBC from 6.6 to 3.7 between homozygous wild type and homozygous minor alleles (Table S3).
SNP/Locus
Allele change
British/European MAF
Disease category
no. genotyped / total (n)
Disease MAF
MAF discordance (t, p-value)
Genetic association with HbF at pre-treatment (β, p-value)
Genetic association with HbF at post-treatment (β, p-value)
Pleiotropic effects (pre-treatment) on hematological parameters (β, p-value)
Pleiotropic effects (post- treatment) on hematological parameters (β, p-value)
rs6545816 (BCL11A)
A → C
0.571
Total hematological malignancies
27/89
0.59
3.33, p < 0.0001
- 0.35, p = 0.44
- 0.09, p = 0.66
0.13, p = 0.46
↓MCV (- 0.64, p = 0.03), ↓MCH (- 0.68, p = 0.01)
Myeloproliferative neoplasm
14/44
0.61
5.22, p < 0.0001
0.44, p = 0.56**
- 0.09, p = 0.79
0.14, p = 0.63
↑Lymphs (0.97, p = 0.04**)
Myeloma
9/33
0.56
-0.40, p =0.47
NA
- 0.4, p = 0.47
NA
↓LDH (- 0.67, p = 0.04)
Myelodysplastic syndrome
4/12
0.63
4.44, p < 0.0001
NA
- 0.43, p = 0.57**
NA
-0.05,p= 0.47
rs1427407 (BCL11A)
G → T
0.121
Total hematological malignancies
35/89
0.06
7.19, p < 0.0001
NA
0.19, p = 0.33
NA
0.02, p = 0.54
Myeloproliferative neoplasm
17/44
0.06
5.95, p < 0.001
NA
- 0.09, p = 0.74
NA
-2.7,p = 0.49
Myeloma
13/33
0.04
7.25, p < 0.0001
NA
0.99, p = 0.002
NA
↑MCV (6.93, p = 0.02)ϯ, ↑PLT (5.42, p = 0.04)ϯ
Myelodysplastic syndrome
5/12
0.1
0.56, p= 0.33
NA
0.56, p = 0.33
NA
-2.59, p = 0.48
rs9494142 (HBS1L-MYB)
T → C
0.187
Total hematological malignancies
42/89
0.24
4.6, p < 0.0001
(5.30, p = 0.03)ϯ
0.46, p = 0.002
-0.08, p = 0.66
-0.16, p = 0.59
Myeloproliferative neoplasm
21/44
0.26
4.93, p < 0.0001
- 0.35, p = 0.81
0.49, p = 0.04
↑PLT (1.72, p = 0.04)
↑Hb (1.85, p = 0.02)
Myeloma
16/33
0.28
5.62, p < 0.0001
0.58, p = 0.42**
0.33, p = 0.25
-1.50, p = 0.45
↑Mo (0.51, p = 0.03)
Myelodysplastic syndrome
12-May
0.2
0.20, p = 0.65
NA
0.35, p = 0.09
NA
↑RBC (0.85, p = 0.004), ↑WBC (0.47, p = 0.04)
rs6920211 (HBS1L-MYB)
T → C
0.187
Total hematological malignancies
49/89
0.31
4.57, p < 0.0001
0.18, p = 0.53
0.35, p = 0.01
-0.04, p =0.68
↑BILI (0.27, p = 0.04), ↓WBC (3.73, p = 0.03)ϯ
Myeloproliferative neoplasm
24/44
0.27
2.98, p < 0.004
- 0.35, p = 0.81
0.41, p = 0.05
↑PLT (1.72, p = 0.04)
↑Hb (1.85, p = 0.02)
Myeloma
18/33
0.25
2.2, p = 0.03
0.70, p < 0.001
0.29, p = 0.32
↓RBC (- 1.28, p = 0.03), ↑LDH (0.97, p = 0.03**)
↑WBC (0.50, p = 0.02), ↓MCHC (- 0.91, p = 0.04)**
Myelodysplastic syndrome
5/12
0.57
11.8, p < 0.0001
0.93, p = 0.25**
0.15, p = 0.82
↑MCH (1.0, p = 0.03**), ↓Neut (- 1.0, p = 0.04**)
↓Neut (- 0.41, p < 0.001)
rs9494145 (HBS1L-MYB)
T → C
0.165
Total hematological malignancies
19/89
0.26
3.53, p < 0.0009
NA
0.12, p = 0.61
NA
↑Lymph (0.54, p = 0.03), ↑BAS (0.88, p = 0.003)
Myeloproliferative neoplasm
13/44
0.19
0.19, p = 0.39
NA
- 0.11, p = 0.82
NA
↑Lymph (0.77, p = 0.02), ↑BAS (0.88, p = 0.003)
Myeloma
3/33
0.33
4.61, p < 0.0001
NA
0.41, p = 0.73**
NA
↑Mo (1.0, p < 0.001)**
Myelodysplastic syndrome
3/12
0.5
9.36, p < 0.0001
NA
NA
NA
NA
rs66650371 (HBS1L-MYB)
I → D
0.209
Total hematological malignancies
45/89
0.32
8.83, p < 0.0001
(5.40, p = 0.03)ϯ
0.41, p = 0.005
-0.04, p = 0.66
↓WBC (5.24, p = 0.009)ϯ
Myeloproliferative neoplasm
21/44
0.31
6.41, p < 0.0001
0.16, p = 0.86
0.46, p = 0.05
↓BILI (- 0.70, p = 0.03)
↓Lymphs (-1.07, p = 0.04)
Myeloma
18/33
0.25
2.47, p = 0.01
0.58, p = 0.42**
0.34, p = 0.21
-0.11, p = 0.45
↑Mo (0.46, p = 0.03)
Myelodysplastic syndrome
6/12
0.58
14.3, p < 0.0001
NA
- 0.004, p = 0.99
NA
↓Neut (- 0.43, p = 0.002)
rs748214 (Xmn1-HBG2)
G → A
0.30*
Total hematological malignancies
38/89
0.24
0.24, p= 0.44
0.35, p = 0.17
0.04, p = 0.82
↓Hb (- 0.67, p = 0.03), ↓Eos (- 0.74, p = 0.04), ↑BILI (0.59, p = 0.01)
↑Retics (0.36, p = 0.03), ↑LDH (0.43, p = 0.01), ↑Hb (-0.78, p = 0.02)
Myeloproliferative neoplasm
19/44
0.18
0.18, p = 0.59
0.45, p = 0.58
0.12, p = 0.67
-0.21, p = 0.53
↑Retics (0.59, p = 0.003), ↑LDH (0.79, p = 0.001)
Myeloma
14/33
0.25
0.25, p = 0.85
0.52, p = 0.48**
- 0.06, p = 0.81
0.36, p = 0.47
↓Hb (4.64, p = 0.03)ϯ
Myelodysplastic syndrome
5/12
0.4
0.40, p = 0.71
NA
0.17, p = 0.64
NA
↑MCV (0.74, p < 0.001), ↓MCH (- 0.51, p = 0.02), ↓PLT (- 0.90, p = 0.03)
Total hematological malignancies (n = 89). Abbreviations: HbF = Fetal hemoglobin; SNP = Single Nucleotide Polymorphism; MAF = minor allele frequency; t = t-test value; β = allele effect/gradient; I = no deletion; D = deletion; PLT = platelets; Hb = hemoglobin; RBC = red blood cell count; MCH = mean corpuscular hemoglobin; MCV = mean corpuscular volume; LDH = lactate dehydrogenase; Neut = neutrophils; BILI = bilirubin; Eos = eosinophils; lymphs = lymphocytes; Mo = monocytes; WBC = white blood cell count; MCHC = mean corpuscular hemoglobin concentration; BAS = basophils; Retics = reticulocytes. Adjusted for age & sex as covariates. Bold p-value denotes statistically significant as Bonferroni-adjusted. MAF discordance determined with unpaired two-tailed t-test; genetic association of HbF determined linear regression analysis; mean HbF by genotype determined with one-way analysis of variance; pleiotropic effects determined with linear regression analysis. Pre-treatment = represents baseline sample prior to commencing HbF-inducing cytotoxic agent; Post-treatment = represents = 3 months post initiation of HbF-inducing cytotoxic agent (Hydroxyurea for Myeloproliferative neoplasm, Lenalidomide for Myeloma, Azacytidine for Myelodysplastic syndrome). *XMN1-HBG2 MAF derived from Thein et al. Control of fetal hemoglobin: new insights emerging from genomics and clinical implications. Human Molecular Genetics 2009; 18(R2): R216 – R223** not adjusted for age and sex due to insufficient sample number NA = not available due to insufficient allele sample number. Confidence intervals provided in supplemental data. ϯ significant on analysis of variance determining effect of genotype on mean HbF levels therefore F-statistic and p-value provided. HbF levels adjusted for age and sex at baseline.
Table 2: Influence of HbF genetic variants on baseline/induced HbF and hematological traits in MPN, Myeloma and MDS by linear regression analysis.
Amongst the MPN on HU group, HMIP variant rs9494142 was observed as significantly associated with induced HbF levels (p = 0.04) (Figure 1a) as well as influencing increase on baseline platelet (PLT) count (p = 0.04) and increasing induced Hb levels (p = 0.02). rs9494142 (HMIP) was associated with 216.2% increase in mean HbF from 0.68 in the TT homozygous wild type to 2.15 in the CC homozygous minor allele although this only trended significance (p = 0.09) (Table S3). Analogous to rs9494142 (HMIP), rs6920211 (HMIP) was also seen to affect different hematological parameters at different stages of treatment shifting from regulation of baseline PLT levels (p = 0.04) to the regulation of induced Hb levels (p = 0.02) 3-months post cytotoxic agent initiation.
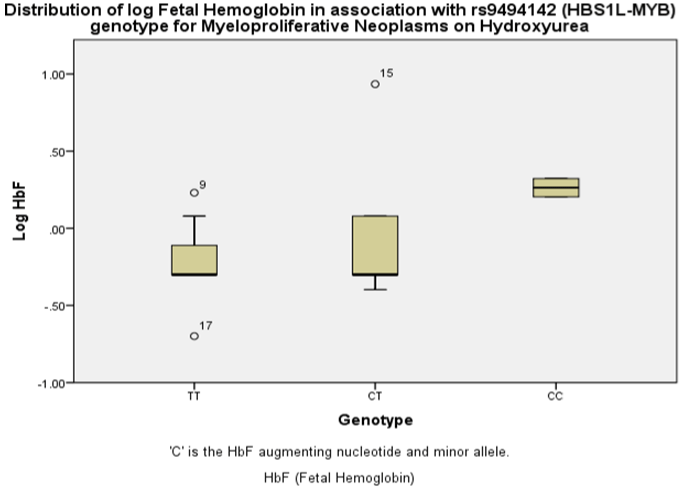
Figure 1a: Association of HbF variant rs9494142 (HBS1L-MYB) with HbF in patients with myeloproliferative neoplasm on Hydroxyurea with linear regression
analysis (p<0.04).
Amongst the Myeloma on Lenalidomide group, rs6920211(HMIP) was significantly associated with baseline HbF (p < 0.001) as well as influencing the decrease of baseline Red Blood Cell count (RBC) (p = 0.03) and the increase of induced WBC (p = 0.02). BCL11A variant rs1427407 was strongly associated with induced HbF levels with Lenalidomide (p = 0.002) (Figure 1b). This variant influenced 373% increase in mean HbF from 0.74 with the homozygous GG allele to 3.5 with the heterozygous minor GT allele (p = 0.009) (Table S3). rs1427407 (BCL11A) was also significantly associated with increased Mean Corpuscular Volume (MCV) (p = 0.02) and increased PLT count (p = 0.04) seemingly negating the thrombocytopenic tendency of Lenalidomide (Table 2 and Table S3). There was 168% increase in mean PLT count from 167.1 with the homozygous GG allele to 448 with the heterozygous minor GT allele (p = 0.04). It was also associated with 11.4% increase in mean MCV (p = 0.02) and 27.3% decrease in Hb between the homozygous and heterozygous genotypes. Xmn1-HBG2 (rs748214) did not achieve statistically significant association with HbF but was associated with decreased Hb (p = 0.03) in Myeloma and increased levels of hemolysis markers (reticulocytes (p = 0.003) and LDH (p = 0.001)) in MPN patients on HU. Xmn1- HBG2 influenced 147.5% increase in mean reticulocyte count from 69.1 with homozygous GG allele to 171 on homozygous AA minor allele (p < 0.001) (Table S3). Decreased Hb (p = 0.03) and increased BILI (p = 0.01) were also significantly associated with Xmn1-HBG2 (rs748214) in addition to decreased eosinophil count (p = 0.04) amongst the total hematological malignancy group. Although, we found no specific genetic association with HbF in patients with MDS on AZA, HbF variant influence on other widespread hematological parameters was very apparent (Table 2). We found no significant association between karyotype and HbF in MDS, neither did we see any significant relationship between JAK2 status in MPN disease subgroups and HbF (data not shown).
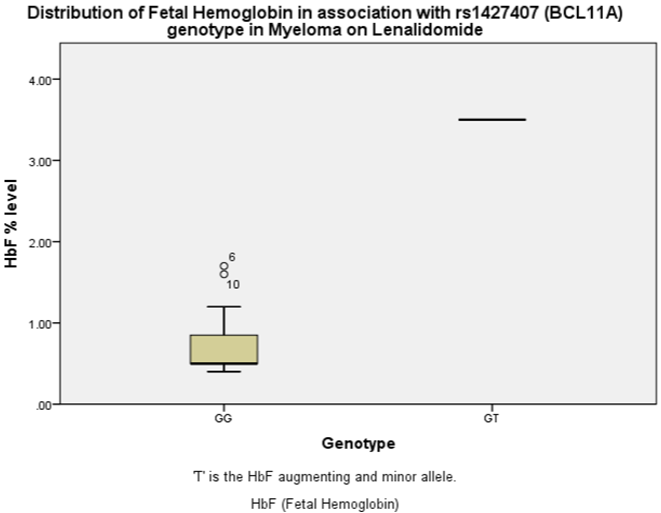
Figure 1b: Association of HbF variant rs1427407 (BCL11A) with HbF in patients with myeloma on Lenalidomide with linear regression analysis (p = 0.002).
HbF Genetic Variants Show Minor Allele Frequency Discordance amongst MPN/Myeloma/MDS Patients
We compared Minor Allele Frequencies (MAF) of HbF variants amongst patients with MPN/Myeloma/MDS with MAF of HbF variants from wider British/European population control data derived from 1000 Genome project. We found that our MPN/myeloma/MDS study population had significantly discordant MAF in comparison to MAF from the control population (Figure 2). MAF aberration was most prominent amongst MDS as shown by the greatest significantly discordant frequencies across 4 HbF variants at HBS1L-MYB and BCL11A loci (Figure 2 & Table 2). MAF amongst MPN had the next largest discordance with MAF aberration across 5 HbF variants at HBS1L-MYB and BCL11A loci. Patients with myeloma had smaller but still significant MAF aberration across 5 HbF variants on HBS1LMYB and BCL11A loci. No significant MAF aberrations were observed with XMN1-HBG2 loci.
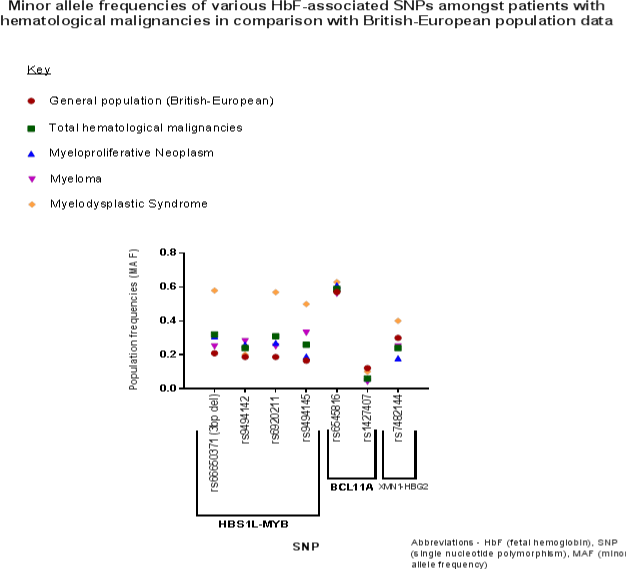
Figure 2: Analysis of minor allele f requencies of HbF genetic variants in hematological malignancies in comparison with wider British-European population genome
data using linear regression and t-test.
Discussion
Genetic studies of HbF can present opportunities for useful insight into hematological patterns of response to treatment and prognostic biomarkers. We report common fetal hemoglobin variants do modify hematological phenotypes in MPN/Myeloma/ MDS patients receiving cytotoxic drugs. HbF was variably expressed in our study group on HbF-inducing agents highlighting degree of genetic influence in treatment response. MDS on AZA group showed highest levels of baseline and induced HbF followed by MPN on HU and Myeloma on Lenalidomide groups respectively. Elevated baseline HbF was seen in 1 in 4 of our hematological malignancy pre-treatment cohort. MDS on AZA group had more than double mean induced HbF levels at 2.36% compared to MPN on HU at 1.06% and threetimes more than the Myeloma patients on Lenalidomide at 0.77%. Our HbF induction profile differs from SCD HbF induction profile studies which illustrate Pomalidomide (Lenalidomide analogue) HbF levels equivalent to or greater than levels seen with HU. [22,23] We also found that our MPN population on HU had less prevalence of HbF responders (28.6%) in comparison to SCD population on HU owing probably to the lower baseline HbF levels comparatively to begin with. [24,25] Nevertheless, it remains impossible to make full comparisons due to the differences in disease populations. The various hematological phenotypes such as anemia and macrocytosis observed in our study population reflect treatment and/or disease specific myelosuppression and stress erythropoiesis. Likewise, we did not include dosage of cytotoxic agent, as this is difficult to compare between the disease sub-group populations. Nevertheless, it is likely, that a dosage effect will be observed between cytotoxic agent and HbF level if the diseases are further studied individually. Similarly, more larger and focused disease phenotype studies should be undertaken to determine HbF quantitative trait loci effect and respective treatment dosage regimens on disease-specific clinical outcomes like achievement of disease control, need for packed RBC transfusions, normalization of hemoglobin concentration or platelet count for MPNs, myeloma response rate as well as event-free survival for MDS.
Whilst the influence of HbF genetic loci which modify hematological phenotype leading to favorable outcomes in SCD & β-thalassemia is well known, there is a distinct lack of comparable evidence evaluating HbF variant influence in patients with hematological malignancies. Lübbert et al. [4] however demonstrated the prognostic value of elevated pre-treatment HbF levels in predicting better outcome for MDS/AML patients receiving decitabine therapy. Developing on this, our study reveals the genotypic significance of HbF and effects on hematological traits amongst patients with hematological malignancy on HbF-inducing cytotoxic therapy thus indicating the potential for these HbF variants in prediction of treatment response. These findings within this small study are further contextually validated by Tapper et al. [26] Genome Wide Association Study (GWAS) of 3437 MPN cases which showed that, in addition to established MPN driver mutations like JAK2 and CALR which predispose to MPN, they also found surprisingly HBS1L-MYB variant rs9376092 as significantly associated with MPN, in particular JAK2V617F-positive ET cases. For JAK2V617F-negative cases, rs9376092 (HBS1L-MYB) achieved significance conditional on CALR and/or MPL mutations. [26] Unfortunately, we were not able to replicate and corroborate this exact Single Nucleotide Polymorphism (SNP) amongst our MPN group as we did not select this SNP to genotype from the outset of the study. However, we can of novel verify another HBS1L-MYB SNP rs9494142 as significantly associated with HbF in our MPN group on HU. Furthermore, in line with Tapper et al. [26] showing influence of HBS1L-MYB variant towards the development of ET amongst MPN patients, our study also highlights PLT involvement with association of increased PLT count and HbF levels (Beta = 0.35, p = 0.04, Table 2) amongst our MPN group on HU. Studies describing SCD HU-associated HbF induction involve numerous complex signal transduction pathways through key genomic variants BCL11A, HBS1L-MYB and SAR1. [27] In our study, a patient with MPN had an intriguing HbF induction to 25% while he was on HU. This patient had Type 2 Diabetes and was also on Metformin (now known to be a potent HbF inducer). [28] SNP assay for this patient revealed he was heterozygous for four HBS1L-MYB variants and homozygous for the mutant allele at the BCL11A (rs6545816) locus.
Thein et al. [11] identified genetic variants at three principal linkage disequilibrium blocks at HMIP blocks 1, 2, and 3 spanning approximately 79kb responsible for 19% of HbF trait variance. HMIP also has the most abundant pleiotropic effects on human hematological traits. [29] The most powerful of these effects are mostly attributable to HMIP-2 variants such as rs9494142, 3bp-del rs66650371 and rs6920211 as these variants reside within a core-enhancer for MYB, a major hematopoietic gene regulator which disrupt binding for key transcription factors like GATA. [30] Our study showed HMIP-2 variants rs9494142 and rs6920211 influencing increased baseline PLT count amongst MPN on HU group. These variants were also seen to affect different hematological parameters at different stages of treatment such as when these HMIP-2 variants switched influence of baseline PLT count to regulation of induced Hb levels 3-months post treatment initiation. Amongst, our total hematological malignancy group, HMIP-2 (rs6920211) was also associated with BILI whilst on HbF-inducing cytotoxic therapy.
HBS1L-MYB variants have been shown to be associated with baseline HbF in African American patients with sickle cell anemia. [14] We confirm another HBS1L-MYB variant rs6920211 as associated with baseline HbF levels amongst Myeloma patients. BCL11A variant rs1427407 was strongly associated with induced HbF levels in this group on Lenalidomide. BCL11A association in Myeloma is shown by previous work from Dulmovits et al. [22] and Kubi-Appiah et al. [31] in Pomalidomide-induced reactivation of HbF through BCL11A. Dulmovits et al. [22] also found HbF reactivation involving Ikaros, which is the degradation target in Myeloma treatment with Pomalidomide. Our study also showed rs1427407 (BCL11A) significantly associated with increased platelet count seemingly withstanding the thrombocytopenic tendency of Lenalidomide. Sub-groups with this variant should be stratified and evaluated for increased risk of thromboembolism amongst patients with Myeloma on Lenalidomide highlighting potential functional significance of rs1427407 (BCL11A) variant on hematological traits. BCL11A loci account for 15% of HbF variance in human populations. [12] Differences in BCL11A expression level of transcript have been recently implicated in disease biology of Acute Myeloid Leukemia and acute phase Chronic Myeloid Leukemia [32,33].
The XMN1-HGB2 (rs782144) locus within the β-globin gene cluster contributes 10% of HbF variance. [12] Although no significant HbF association was found, XMN1-HBG2 (rs782144) did influence various hematological parameters including increased markers of hemolysis e.g. LDH and reticulocytes amongst MPN patients on HU in our study. XMN1-HBG2 (rs782144) was also associated with reduction in PLT count amongst MDS patients on AZA making this a useful line of investigation for thrombocytopenia within this disease group.
Our study of novel shows that these major HbF-associated variants may also be implicated in the disease biology of patients with MPN/Myeloma/MDS evidenced by MAF discordance when compared to wider British/European population MAF data derived from 1000 genome project. Although our sample data is small, this fits and corroborates findings from Tapper et al. [26] MPN GWAS study showing HBS1L-MYB affecting MPN disease biology predisposing to the development of ET. The marker found associated in this paper (rs9376092) tracks the same causative genetic variant (3-bp deletion) as the markers used in the present study. [34] JAK2 is also known for its pleiotropic effect on various hematological parameters [6], as such, we analyzed the association between JAK2 status of MPN disease subtypes and influence on HbF levels but found no significant association. We did note however that JAK2V617F-positive ET patients had most elevated HbF levels among MPN disease sub-groups but this did not achieve statistical significance possibly due to our small sub-group sample size. Amongst MDS patients, a previous study had identified higher HbF levels amongst those with karyotypic abnormality. [35] We, however found no association between karyotype and HbF in MDS.
Whilst 89 patients is reasonable statistical study strength, disease sub-group analysis have smaller sample sizes and smaller numbers genotyped particularly for our MDS group as such it is possible that not all statistically significant associations could be identified within this study. Consequently, larger powered, prospective genotype-phenotype association studies are warranted. Despite our small sample size, the data validates previous studies and highlights that the common HbF variants influence HbF and hematological traits in MPN, Myeloma and MDS and suggests that these variants are involved in disease biology and treatment response for these conditions. Consequently, illustrating that HbF serves as a conduit bio-marker for the presence of genetic variants that influence the hematological response in these diseases.
Acknowledgements
We would like to thank The British Society of Haematology (BSH) for providing financial support for this study. Author NI was the recipient of a BSH Early Stage Research start-up grant and was a UK NIHR Academic Clinical Fellow in Haematology at the time of the study. This study also received an American Society of Hematology (ASH) Abstract Achievement Award.
We thank Dr Muzaffar Malik for statistical advice with the study. We thank Tim Futter for ethics and administrative support to this study at his time at King’s College London.
We would like to also thank the patients at the haematology clinic at Brighton & Sussex University Hospitals NHS Trust for their kind participation in this study. We also thank the staff at the BSUH haematology department for their help in facilitating this study.
Author Contribution
NI, TC, SLT, and SM conceived and designed the study. NI performed the research. NI, AP, HH, DJ, and TC recruited the patients. NI, HR and SM performed the genotyping. AB and HR performed the FACS. NI, AP, HH, DJ and DM curated the data. NI and SM performed the data and genetic analyses. NI wrote the manuscript. All authors reviewed the manuscript. TC, SLT and SM provided critical review for the manuscript and provided senior supervision the overall study.
Conflict of Interest
The authors declare no conflict of interest or competing financial interest.
References
- Thein SL, Menzel S, Lathrop M, Garner C. Control of fetal hemoglobin: new insights emerging f Rom genomics and clinical implications. Hum Mol Genet. 2009; 18: R216-23.
- Eridani S, Mosca A. Fetal hemoglobin reactivation and cell engineering in the treatment of sickle cell anemia. J Blood Med. 2011; 2: 23-30.
- Bauer DE, Kamran SC, Orkin SH. Reawakening fetal hemoglobin: prospects for new therapies for the β – globin disorders. Blood. 2012; 120: 2945-53.
- Lübbert M, Ihorst G, Sander PN, Bogatyreva L, Becker H, Wijermans PW et al. Elevated fetal haemoglobin is a predictor of better outcome in MDS/AML patients receiving 5- aza-2'-deoxycytidine (decitabine). Br J Haematol. 2017; 176: 609-17.
- Ware RE, Eggleston B, Redding-Lallinger R, Wang WC, Smith-Whitley K, Daeschner C et al. Predictors of fetal hemoglobin response in children with sickle cellanemia receiving hydroxyurea therapy. Blood. 2002; 99: 10-4.
- Chami N, Chen MH, Slater AJ, Eicher JD, Evangelou E, Tajuddin SM et al. Exome genotyping identifies pleiotropic variants associated with red blood cell traits. Am J Hum Genet. 2016; 99: 8-21.
- Garner C, Tatu T, Reittie JE, Littlewood T, Darley J, Cervino S et al. Genetic inf luences on F cells and other hematologic variables: a twin heritability study. Blood. 2000; 95: 342-6.
- Sankaran VG, Orkin SH. The switch f Rom fetal to adult hemoglobin. Cold Spring Harb Perspect Med. 2013; 3: a011643.
- Tumburu L, Thein SL. Genetic control of erythropoiesis. Curr Opin Hematol. 2017; 24: 173-82.
- Alter BP, Rosenberg PS, Day T, Menzel S, Giri N, Savage SA et al. Genetic regulation of fetal haemoglobin in inherited bone marrow failure syndromes. Br J Haematol. 2013; 162: 542-6.
- Thein SL, Menzel S, Peng X, Best S, Jiang J, Close J et al. Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 inf luencing fetal hemoglobin levels in adults. Proc Natl Acad Sci U S A. 2007; 104: 11346-51.
- Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S et al. A QTL inf luencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet. 2007; 39: 1197-9.
- Khanh T et al. The XmnI G γ polymorphism inf luences hemoglobin F synthesis contrary to BCL11A and HBS1L- MYB SNPs in a cohort of 57 β-thalassemia intermedia patients. Blood Cells Mol Dis. 2010; 45: 124-7.
- Akinsheye I, Alsultan A, Solovieff N, Ngo D, Baldwin CT, Sebastiani P et al. Fetal hemoglobin in sickle cell anemia. Blood. 2011; 118: 19-27.
- Bauer DE, Orkin SH. Update on fetal hemoglobin gene regulation in hemoglobinopathies. Curr Opin Pediatr. 2011; 23: 1-8.
- Lettre G, Sankaran VG, Bezerra MA, Araújo AS, Uda M, Sanna S et al. DNA polymorphisms at the BCL11A, HBS1L-MYB, and beta-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci U S A. 2008; 105: 11869-74.
- Banan M, Bayat H, Azarkeivan A, Mohammadparast S, Kamali K, Farashi S et al. The XmnI and BCL11A single nucleotide polymorphisms may help predict hydroxyurea response in Iranian β-thalassemia patients. Hemoglobin. 2012; 36: 371-80.
- Campbell PJ, Green AR. The myeloproliferative disorders. N Engl J Med. 2006; 355: 2452-66.
- Gryn J, Zeigler ZR, Shadduck RK, Lister J, Raymond JM, Sbeitan I et al. Treatment of myelodysplastic syndromes with 5-azacytidine. Leuk Res. 2002; 26: 893-7.
- Holstein SA, McCarthy PL. Immunomodulatory drugs in multiple myeloma: mechanisms of action and clinical experience. Drugs. 2017; 77: 505-20.
- Singh A, Xu YJ. The cell killing mechanisms of hydroxyurea. Genes (Basel). 2016; 7: 99.
- Dulmovits BM, Appiah-Kubi AO, Papoin J, Hale J, He M, Al-Abed Y et al. Pomalidomide reverses -globin silencing through the transcriptional reprogramming of adult hematopoietic progenitors. Blood. 2016; 127: 1481- 92.
- Meiler SE, Wade M, Kutlar F, Yerigenahally SD, Xue Y, Moutouh-de Parseval LA et al. Pomalidomide augments fetal hemoglobin production without the myelosuppressive effects of hydroxyurea in transgenic sickle cell mice. Blood. 2011; 118: 1109-12.
- Chand AR, Xu H, Wells LG, Clair B, Neunert C, Spellman AE et al. Are there true non-responders to hydroxyurea in sickle cell disease? a multiparameter analysis. Blood. 2014; 124: 4073.
- Green NS, Barral S. Genetic modifiers of HbF and response to hydroxyurea in sickle cell disease. Pediatr Blood Cancer. 2011; 56: 177-81.
- Tapper W, Jones AV, Kralovics R, Harutyunyan AS, Zoi K, Leung W et al. Genetic variation at MECOM, tert, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat Commun. 2015; 6: 6691.
- Pule GD, Mowla S, Novitzky N, Wiysonge CS, Wonkam A. A systematic review of known mechanisms of hydroxyurea-induced fetal hemoglobin for treatment of sickle cell disease. Expert Rev Hematol. 2015; 8: 669-79.
- Zhang Y, Weiss M, Sumazin P, Sheehan VA. Metformin induces FOXO3- dependent fetal hemoglobin production in primary erythroid cells. Blood. 2016; 128.
- Menzel S, Jiang J, Silver N, Gallagher J, Cunningham J, Surdulescu G et al. The HBS1L-MYB intergenic region on chromosome 6q23.3 inf luences erythrocyte, platelet, and monocyte counts in humans. Blood. 2007; 110: 3624-6.
- Stadhouders R, Aktuna S, Thongjuea S, Aghajanirefah A, Pourfarzad F, van Ijcken W et al. HBS1L-MYB intergenic variants modulate fetal hemoglobin via long -range MYB enhancers. J Clin Invest. 2014; 124: 1699-710.
- Appiah-Kubi AO, Blanc L, Singh SA, Didier S, Dsilva S, Chan KWH et al. Pomalidomide augments fetal hemoglobin production in primary erythroid cells by a novel mechanism modulating BCL11A but not KLF-1. Blood. 2013; 122: 314.
- Tao H, Ma X, Su G, Yin J, Xie X, Hu C et al. BCL11A expression in acute myeloid leukemia. Leuk Res. 2016; 41: 71-5.
- Yin J, Zhang F, Tao H, Ma X, Su G, Xie X et al. BCL11A expression in acute phase chronic myeloid leukemia. Leuk Res. 2016; 47: 88-92.
- Menzel S, Rooks H, Zelenika D, Mtatiro SN, Gnanakulasekaran A, Drasar E et al. Global genetic architecture of an erythroid quantitative trait locus, HMIP- 2. Ann Hum Genet. 2014; 78: 434-51.
- Craig JE, Sampietro M, Oscier DG, Contreras M, Thein S. Myelodysplastic syndrome with karyotype abnormality is associated with elevated F-cell production. Br J Haematol. 1996; 93: 601-5.