
Case Report
Ann Hematol Oncol. 2022; 9(5): 1407.
Non-Metastatic Prostate Cancer with Severe Anemia: Case Report and Literature Review
Li A, Ji Y, Shen K, Ma Z, Liu B, Su R, Zhang W, Wang Q* and Xue W*
Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, China
*Corresponding author: Qi Wang & Xue W, Department of Urology, Renji Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China
Received: August 24, 2022; Accepted: September 24, 2022; Published: October 01, 2022
Abstract
Prostate cancer is one of the most common tumors of male urinary system and nearly causing any symptoms or signs in early stages. Complication as anemia has been rarely reported in non-metastatic prostate cancer. We present herein an extremely rare case of a local prostate cancer occurring in a 76-yearold man with a complication of severe anemia. As he was diagnosed of prostate cancer, he presented rising severe anemia with the hemoglobin level even down-regulated to 47 g/L. There has been no evidence of tumor metastasis to bone or bone marrow which mainly cause anemia in prostate cancer, supported by emission computed tomography and prostate specific membrane antigen positron emission tomography/computed tomography. According to his hematological tests, he was diagnosed with iron deficiency anemia. Despite the poor responsibility to erythropoietin and blood transfusion during the whole therapy period, his hemoglobin level gradually normalized 4 months after a robot-assisted radical prostatectomy with neoadjuvant endocrine therapy. To our knowledge, there was no such case of severe anemia accompanied with local prostate cancer ever described in the English literature. The case presentation is followed by a general discussion with an emphasis on the diagnosis and differential diagnosis. A review of literature regarding hepcidin and prostate cancer is also discussed.
Keywords: Non-metastatic prostate cancer; Anemia; Hepcidin; Case report and literature review
Introduction
Prostate cancer is the second most common cancer in males [1]. The local tumor is characterized by the symptom of trouble urinating, decreased force in the stream of urinating, blood in semen and discomfort in the pelvic area. The metastatic pattern of advanced prostate cancer is bone pain as the bone tissue is the dominant metastatic site. Other sites of metastases include lymphatic nodes, liver, brain, retroperitoneum, kidney, adrenal gland and bone marrow [2]. Among them, bone marrow, accounts for extreme little metastases, can induce abnormal hemogram and anemia.
Hereby, we report an exceptionally rare case of non-metastatic prostate adenocarcinoma accompanied with severe anemia. This is considered the first case reported in the literature. Moreover, we further discuss the clinical, histological, immunohistochemical finding and provide a review of available literature.
Case Presentation
A 76-year-old man with a long history of mild anemia (hemoglobin level of 107 g/L) for ren years, with no necessary treatment, was found with the elevated PSA level as 5.85 ng/mL. As part of evaluation, his pelvic Magnetic Resonance Imaging scan (MRI) achieved the result of prostate cancer. Biopsy revealed poorly differentiated ductal adenocarcinoma with Gleason Score 3+4=7, and then his hemoglobin level was found decreasing to 50 g/L gradually (Figure 1). Bone marrow aspiration revealed overactivated nucleated cell proliferation, hyperplastic neutrophils, reduced erythroid ratio and increased platelet ratio. Moreover, his bone marrow iron staining showed that extracellular iron was moderate positive (++) and iron granulocytes accounting for 15%. Blood chemistry and flow cytometry disclosed no obvious abnormal results. He denied bone pain, chills, fever and other major systemic diseases. Since there was no clarified cause of anemia, with malignant hematological diseases excluded, he was diagnosed as cancer related iron deficiency anemia. He underwent symptomatic therapy as blood transfusion and erythropoietin agents, whereas his hemoglobin level was maintained at 60 g/L.
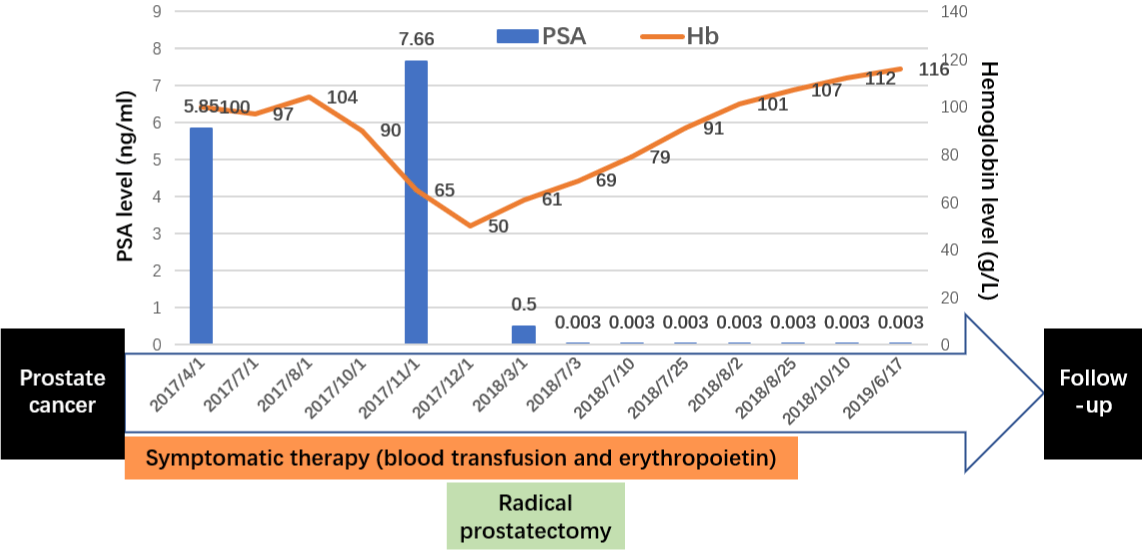
Figure 1: Timeline of the patient.
The patient showed no significant metastases with examined prostate specific membrane antigen positron emission tomography/ computed tomography and bone scan emission computed tomography (Figure 2). His PSA level varied from 5-7 ng/mL after undergoing a 3-month neoadjuvant androgen deprivation therapy (Goserelin 10.8 mg Subq). Eight months after the diagnosis, he underwent robot-assisted radical prostatectomy successfully. The tumor tissue was observed exhibiting normal characteristics, no obviously enlarged lymph nodes or metastasis found during the operation. The immunohistochemical characteristics of tumor disclosed positive PSA, positive AMACR, negative P63, negative 34βE12, negative Erg, positive AR, positive NKX3.1 and 1% positive Ki67 (Figure 3). The instant post-operative PSA level was 0.5 ng/mL and the hemoglobin level was 61g/L. Follow-up serum PSA levels were all ≤0.003 ng/mL for one, three, six, nine and twelve months after surgery. The patient showed irresponsibility to the symptomatic therapy all over the therapy. However, his hemoglobin level gradually normalized in three months after the surgery that eliminate the tumor burden.
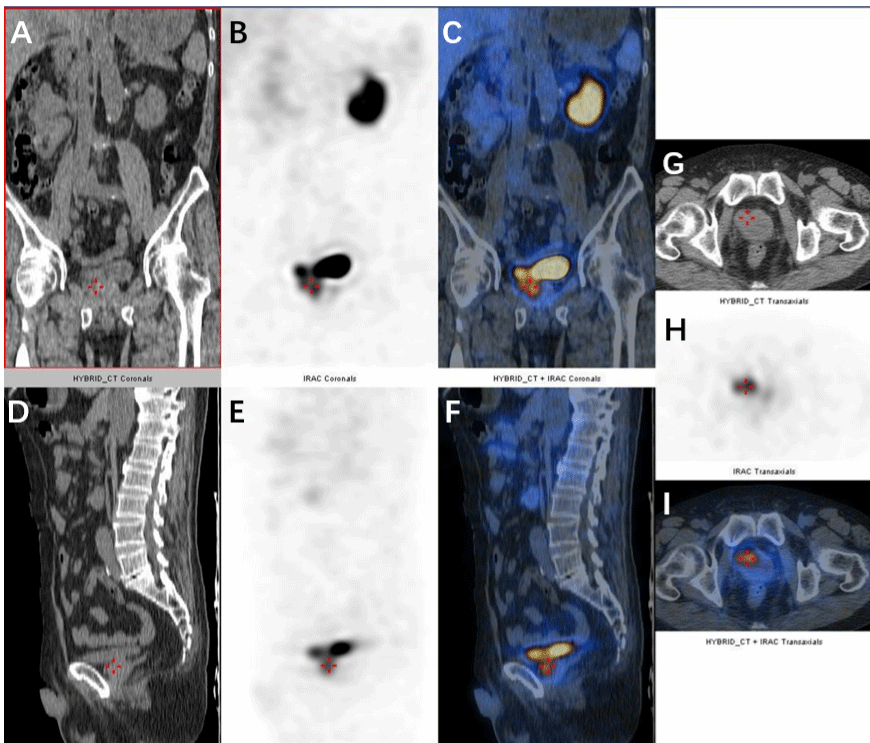
Figure 2: Prostate specific membrane antigen Positron emission tomography/computed tomography (PSMA PET/CT) coronal, sagittal and transverse view.
Coronal view: (A) Non-contrast CT scan of the prostate. (B) PSMA PET/CT scan of the prostate. (C) Fused PSMA PET/CT scan of the prostate. Sagittal view: (D)
Non-contrast CT scan of the prostate. (E) PSMA PET/CT scan of the prostate. (F) Fused PSMA PET/CT scan of the prostate. Transverse view: (G)Non-contrast
CT scan of the prostate. (H) PSMA PET/CT scan of the prostate. (I) Fused PSMA PET/CT scan of the prostate.
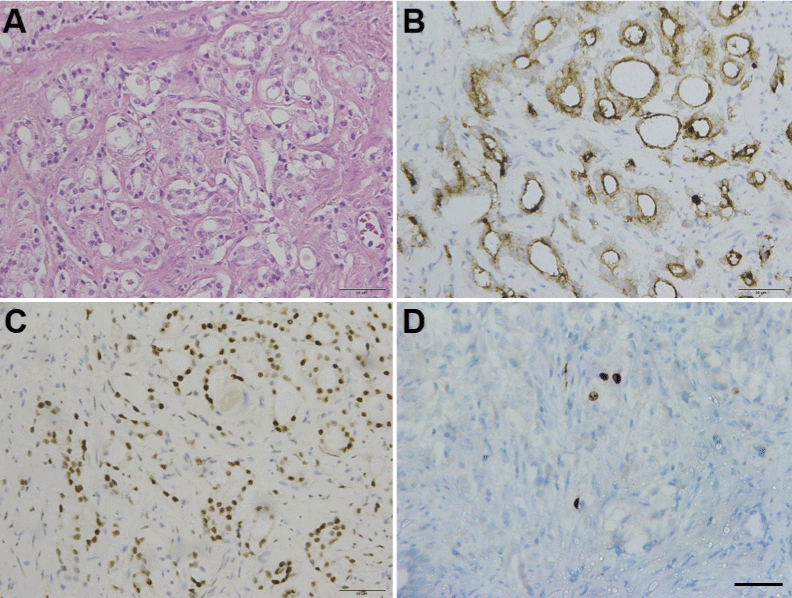
Figure 3: Immunohistochemistry staining of the prostate.
(A) H&Ex20. Carcinoma of prostate show malignant glands with enlarged
nuclei, prominent nucleoli. (B) PSAx20. Malignant glands are positive for
prostate specific antigen (PSA). (C) ARx20. Malignant glands are positive for
androgen receptor. (D) Ki67x20. Malignant glands accounts for less than 1%
positive of Ki67. Scale bars, 50μm.
Discussion
Mild anemia is the most common complication occurring in the prostate cancer patients, leading reduced survival rate. In a systematic review involving 60 selected papers, one-third of the patients were diagnosed as anemic, with the median survival decreased by 20–43% while higher hemoglobin levels correlated with improved outcome measures [3]. Therefore, we look up the literature associated with prostate cancer related anemia, found out the common causes of cancer related anemia [4].
Chemotherapy Induced Anemia
Patients were found anemia during chemotherapy, especial leading to myelosuppressive disease, thrombocytopenia and blood loss.
Anemia Secondary to Cancer
Failure with production of red blood cell: functional iron deficiency, hematological malignancies like MDS, solid tumor bone marrow infiltration, and pure red-cell aplasia.
Overactivation of destruction: hemolysis and hemophagocytosis.
Abnormal blood loss: frequent phlebotomy, mucocutaneous bleeding and gastrointestinal bleeding.
Chronic Kidney Disease in Cancer
Primary chronic kidney disease secondary to cancer or cancer treatment.
In our presented case, it is essential to differential diagnosis and thus approach an appropriate treatment, since the treatments for different types of anemia are quite different.
Myelodysplastic Syndrome (MDS)
MDS is characterized by dysplasia and cytopenia, with morphologic evidence of significant dysplasia. Detection of certain chromosomal abnormalities help distinguish MDS, however, the detection of MDS’s 18 common gene mutation (ASXL1/ CBL/ DNMT3A/ EZH2/ ETY6/ IDH1/ IDH2/ JAK2/ KRAS/ NRAS/ RUNX1/ SF3B1/ THT2/ TP53/ SETBP1/ SRSF2/ U2AF1/ ZRSR2) in the patient with Panel1 genes revealed no hot spot mutations.
Acute Myelocytic Leukemia (AML), Non-Hodgkin Lymphoma (NHL) and High Risked MDS
Bone marrow biopsy showed that CD34+ primordial/naive cells were in normal range (0.2%), and the relative proportion of granulosa was normal in flow cytometry. The immunophenotypes of CD11b, CD13, CD15, and CD16 showed no obvious expression disorder.
Hemolytic Anemia
The patient showed no symptom of hematuria, fever, jaundice and splenomegaly. The Ham’s test and Coomb’s test were negative, while the blood chemistry revealed no elevated hemoglobinemia and hyperbilirubinemia.
Paraneoplastic Syndrome
Paraneoplastic syndrome associated with prostate cancer includes endocrine manifestations, neurological signs, dermatological diseases, etc. Among them, neurological symptoms are the commonest, capable of inducing paraneoplastic cerebellar degeneration, Paraneoplastic Encephalomyelitis (PEM), marginal encephalitis, and Subacute Sensory Neuron disease (SSN) [5]. Paraneoplastic syndromes found in prostate cancer patients were in advanced and metastatic stages, while most of them (70%) complained the paraneoplastic syndrome as the first symptom. Some of paraneoplastic syndrome could be easily detected by serum markers, including Hu antibodies (74% positive) and other antibodies (e.g., Yo, CV2/ CRMP5 and VGCC antibodies) (a few Positive). The patient in the case was an early-stage local prostate cancer displaying no other common paraneoplastic symptoms. As a result, there is no sufficient evidence for this diagnosis.
Bone Marrow Metastasis
The serological test, bone marrow smear and bone marrow biopsy can help diagnose bone marrow metastases. In this case, no carcinomatous cells were found in any tests. Additionally, ECT and PSMA PET/CT excluded bone tissue metastases.
Side Effects Induced by Endocrine Therapy
Endocrine therapy, one of the comprehensive treatment of patients with prostate cancer, may leading to anemia as a common complication. Endocrine therapy functioned as an antagonist to serum testosterone, as castrated by androgen-blocking drugs. Since testosterone is the most important androgen in men, the lack of testosterone may cause a decrease in the level of erythropoietin, leading to a descending in serum red blood cell count and hemoglobin levels. In this case, the low hemoglobin level occurred much earlier than the patient’s endocrine therapy.
General Condition
The patient had normal appetite and diet, good nutritional status and no significantly reduced body weight. He had taken gastrointestinal microscopy which revealed that there was no obvious active bleeding detected.
Results
The severe anemia accompanied with local prostate cancer in this case was caused by multiple factors. Indeed, as suggested in the prior described differential diagnosis, it is reasonable to consider that the anemia condition is closely related to the iron metabolism. Since the change of iron metabolism have the function of increasing serum levels of inflammatory cytokines, activating hepatic synthesis of hepcidin dependent of IL-6 and IL-1, blocking iron mobilization and absorption from hepcidin [6]. To the best of our knowledge, there was no other prostate cancer related severe anemia (hemoglobin level was as low as 47g/L) case reported before. Herein, we post a literature review consisting of two components, abnormal cytokine levels (IL-6, TNF-a, INF-γ) and over expressed hepcidin.
Literature Review
Herein, we aim to explore the possibilities for the severe anemia happens in this patient with prostate cancer. We comprehensively look up the relevant case report and find out that there is almost no report on similar severe anemia condition happening to a prostate cancer patient. Hence, we suggest that the inflammation and tumor status in prostate cancer cause abnormally released cytokines, e.g., IL-6 (interlukin-6) [7], INF-γ, and TNF-a [8]. Note that hepcidin [9] causes the anemia. These cytokines and hepcidin are involved in the survival, growth and metastasis of tumor tissue, on the other hand, they also have the effect of inhibiting bone marrow growth and regulating iron metabolism in the body, resulting in hypoleukemia and iron deficiency anemia. Details on hepcidin and anemia are discussed in the following sections.
Section 1: Iron Homeostasis
Duodenal enterocytes absorb dietary iron and exports the iron supply into the circulation to be utilized for cellular processes (e.g., erythropoiesis). Reticuloendothelial macrophages, recycling iron from senescent erythrocytes, supply most of the iron demanded by the bone marrow for erythropoiesis [11]. Intracellular iron is also stored with ferritin in the liver, and where it accounts for a third of the body’s iron stores. Intracellular iron can be exported from the hepatocytes when needed [12].
Section 2: Hepcidin is Overexpressed in Patients with Prostate Cancer
Serum hepcidin level in patients with prostate cancer is significantly higher than that in patients with normal prostate or benign prostate hyperplasia. This could be caused by multiple factors. Firstly, elevated inflammatory cytokine in tumor patients can stimulate the synthesize synthesis and secretion of hepcidin in the liver [13]. Secondly, the prostatic epithelial cells can synthesize and secrete hepcidin, while the prostate cancer cells are likely to oversynthesize and secrete hepcidin. Third, the iron metabolism disorder in the prostate cancer patient may also lead to abnormal levels of hepcidin in reverse [14].
Section 3: Regulatory Functions of Hepcidin
Hepcidin has the functions of binding to and initiating the degradation of ferroportin, the only known iron exporter, distributing on the cell surface of duodenal enterocytes, macrophages and hepatocytes. Thus, overexpressed hepcidin and downregulated ferroportin will suppress the transportation of cellular iron into the plasma from these cells [15].
Section 4: The Signaling Pathway of Hepcidin Regulation
The prostate cancer can stimulate autoimmune system to activate CD3+T cells, monocytes and lipopolysaccharide through immunoregulatory mechanisms, thereby generating interferongamma (IFN-γ), tumor necrosis factor-a (TNF-a), interleukin-1 (IL-1), interleukin-6 (IL-6) and other inflammatory factors. These inflammatory factors can promote the high expression of hepcidin synthesized by hepatocytes through different pathways including:
1. IL-6-STAT3 Pathway
IL-6, an inflammatory factor involved in mediating prostate tumors by destroying the immune response and regulating the tumor microenvironment. It can facilitate prostate cancer cell proliferation and inhibit apoptosis via various signaling pathways. In inflammatory setting, IL-6 is released. It binds to its receptor and then forms a complex further activating the Janus tyrosine hormone family kinase 1/2 (JAKs1/2). Subsequently, it phosphorylates the signal transduction and transcriptional activator (STAT), mainly STAT3. The STAT3 protein is confirmed as the promoter of Hepcidin in the study of Writing [16]. As a result, IL-6 can up-regulate hepcidin expression. Furthermore, IL-6 can also promote the proliferation and inhibit the apoptosis of prostate cancer cells through the mitogenactivated protein (MAP) kinase--extracellular signal-regulated kinase 1/2 (ERK1/2) pathway and the phosphoinositide 3-kinase (PI3-K) pathway.
2. BMP6-HJV-SMAD Pathway
BMP, a bone morphogenetic protein, acts as a TGF family cytokine. It is capable of regulating serum hepcidin level via the Hjv- BMP-SMAD signaling pathway [17]. With the high expression of various BMP proteins in prostate cancer cells, the hepatic synthesis of hepcidin can be regulated, which are primarily BMP4/6/7. When systemic iron meets insufficiency, circulating BMP6 binds to transmembrane BMP receptor type I (BMP-RI), type II (BMPRII), and BMP co-receptor hemagglutinin (HJV) to form a complex structure on the liver membrane and to activate the SMAD signaling cascade. Subsequently, the activated intracellular SMAD1/5/8 protein is combined with the common mediator SMAD4 and then transferred to the nucleus by targeting the BMP-responsive elements (BMP-RES) on the hepcidin promoter to induce hepcidin expression.
3. Wnt Pathway
The activation of Wnt signaling pathway has been shown to be present in numerous cancers, including prostate cancer. Lia Tesfay’s study suggested that the transfection of prostate cancer cells with a 2.7 kb hepcidin promoter-driven luciferase reporter gene showed hepcidin a direct downstream target of Wnt pathway [14]. As a result, it was demonstrated that the Wnt signaling pathway also up-regulates the high expression of hepcidin in prostate cancer cells.
Section 5: The Relation Between Hepcidin and Anemia
Hepcidin is a circulating antimicrobial peptide mainly synthesized in the liver and has recently been verified as a vital factor regulating iron uptake in the small intestine and mobilizing iron from macrophage cells and liver cell storage. The imbalance of hepcidin acts as a causative factor of anemia by disrupting iron homeostasis. For instance, the overload of iron can cause hereditary hemochromatosis and iron-loaded anemia due to hepcidin deficiency. On the other hand, excessive hepcidin may lead to a low iron level, followed by the processing of inflammation, development of disease, infection, some cancer and anemia of chronic diseases [18].
Hepcidin regulates the iron metabolism mainly through two pathways:
1. Hepcidin, a negative regulator of iron metabolism, binds to its receptor ferroprotin forming a Hepcidin-Ferroprotin Complex, which can induce the iron internalization and degrade in the cytosol, decrease the iron concentration on the cell membrane and immobilize iron in the cell. Internally, it can hinder the absorption and transportation of iron in intestinal mucosal cells. When hepcidin is highly expressed in prostate cancer patients, the transferrin function of ferroprotinare too decreased to meet the needs, which leads to the inability of iron in the intestinal mucosa to be excreted, and the iron absorption to be reduced. They furtherly will cause Iron Deficiency anemia (IDA).
2. Ferroportin, a receptor of hepcidin, is widely distributed in reticuloendothelial cells and macrophages. The overexpression of hepcidin inhibits the expression of ferroportin, resulting in the disfunction of iron transportation, which mainly through the transmembrane transport of ferroportin protein. This is crucially important so as to influence the supplement of iron in the blood, thus can also cause iron deficiency anemia.
Section 6: The Relation between Cytokines and Anemia
Besides hepcidin, inflammatory response factors also play an important role in anemia in prostate cancer patients. Here we will briefly introduce three cytokines, including TNF-a, INF-γ and erythropoietin. Studies have shown that under the effect of TNF-a and INF-γ, the responsiveness of bone marrow erythroid towards EPO is reduced, and the function of EPO synthesized by the kidney is also inhibited. The combination effect of these two factors lead to a decrease in erythropoiesis and the occurrence and development of anemia.
Furthermore, TNF-a is an inflammatory cytokine stimulator involved in systemic inflammation and acute phase reactions. Excessive TNF-a in the serum of patients with prostate cancer can cause Hematopoietic Stem Cell (HSC) damaged, includes affecting the survival, homing and proliferation process of bone marrow [19].
Discussion
We reported a rare case of non-metastatic prostate cancer with severe anemia, in this case, the patient showed no significant improvement in anemia status after the treatment with EPO agents and blood transfusion symptomatic treatment, revealing that it may be irresponsible to erythropoietin because of the abnormal release of TNF-a, IL-1 and IL-6 in prostate cancer patients [8]. The iron metabolism tests, which cover positive extracellular iron (++) in bone marrow iron staining and iron myelocytes over 15%, suggest that the iron metabolism abnormalities are a critical factor inducing iron deficiency anemia, which is associated with the high expression of hepcidin. As a result, hepcidin overexpression is found causing the iron metabolism disorders in the body.
Several researchers suggest that the growth, angiogenesis and metastasis of prostate cancer cells are related to iron metabolism [20]. Moreover, iron is a necessity for normal human growth and exercise, especially the raw material of erythropoiesis in synthesizing the heme.
Hepcidin is a cyclic peptide hormone synthesized in the liver, and it is critical in the body’s absorption of iron and regulation of iron redistribution [14]. It is an essential protein regulating intestinal iron absorption and distribution of systemic iron including reticuloendothelial macrophages [11].
As hepcidin is highly expressed in patients with prostate cancer, it may act as an important factor that regulating prostate cancer and anemia through the iron metabolism in the body.
After overviewing the relevant literatures, the relevance of cytokines in prognosis and complications in prostate cancer patients is quite precise, and it can be a good guideline and marker for the prevention, treatment and prognosis in prostate cancer. The level of TNF-a, IL-1, IL-6 and other laboratory tests, as well as the irresponsiveness of EPO agents in tumor patients can be predicted for the necessity for erythropoietin and iron supplement treatment. Additionally, detecting serum levels of BMP-6 and hepcidin will help estimate the further survival and predict the prognose of patients.
Acknowledgments
This study was supported by National Natural Science Foundation of China (81702840, 81702542, 81572536, 81672850, 81772742), Science and Technology Commission of Shanghai Municipality (16411969800), Shanghai Shenkang Hospital Development Center (16CR3049A), Shanghai Jiao Tong University (YG2017MS47, YG2017MS52).
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394- 424.
- Gandaglia G, Abdollah F, Schiffmann J, Trudeau V, Shariat SF, Kim SP, et al. Distribution of metastatic sites in patients with prostate cancer: A populationbased analysis. Prostate. 2014; 74: 210-216.
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001; 91: 2214-2221.
- Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancerrelated anemia. Am J Hematol. 2014; 89: 203-212.
- Hong MK, Kong J, Namdarian B, Longano A, Grummet J, Hovens CM, et al. Paraneoplastic syndromes in prostate cancer. Nat Rev Urol. 2010; 7: 681- 692.
- Colloca G, Venturino A, Vitucci P, Gianni W. Management of anaemia in prostate cancer. Cancer Invest. 2010; 28: 280-288.
- Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU Int. 2014; 113: 986-992.
- Pavese I, Satta F, Todi F, Di Palma M, Piergrossi P, Migliore A, et al. High serum levels of TNF-a and IL-6 predict the clinical outcome of treatment with human recombinant erythropoietin in anaemic cancer patients. Ann Oncol. 2010; 21: 1523-1528.
- Zhao B, Li R, Cheng G, Li Z, Zhang Z, Li J, et al. Role of hepcidin and iron metabolism in the onset of prostate cancer. Oncol Lett. 2018; 15: 9953-9958.
- Finch CA, Huebers H. Perspectives in iron metabolism. N Engl J Med. 1982; 306: 1520-1528.
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011; 117: 4425- 4433.
- Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010; 95: 501-504.
- Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol. 2012; 87: 392-400.
- Tesfay L, Clausen KA, Kim JW, Hegde P, Wang X, Miller LD, et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015; 75: 2254-2263.
- De Domenico I, Ward DM, Langelier C, Vaughn MB, Nemeth E, Sundquist WI, et al. The molecular mechanism of hepcidin-mediated ferroportin down regulation. Mol Biol Cell. 2007; 18: 2569-2578.
- Ye L, Kynaston HG, Jiang WG. Bone metastasis in prostate cancer: molecular and cellular mechanisms (Review). Int J Mol Med. 2007; 20: 103-111.
- Silvestri L, Nai A, Dulja A, Pagani A. Hepcidin and the BMP-SMAD pathway: An unexpected liaison. Vitam Horm. 2019; 110: 71-99.
- Arezes J, Nemeth E. Hepcidin and iron disorders: new biology and clinical approaches. Int J Lab Hematol. 2015; 37: 92-98.
- Du W, Erden O, Pang Q. TNF-a signaling in Fanconi anemia. Blood Cells Mol Dis. 2014; 52: 2-11.
- Msaouel P, Pissimissis N, Halapas A, Koutsilieris M. Mechanisms of bone metastasis in prostate cancer: clinical implications. Best Pract Res Clin Endocrinol Metab. 2008; 22: 341-355.