
Research Article
J Hepat Res. 2014;1(1): 1001.
"Transparent" Liver
Fanpu Ji1,2, Hongan Xue2, Weili Min3, Hong Deng2, and Zongfang Li1*
1Department of General Surgery, Xi'an Jiaotong University, China
2Department of Infectious Diseases, Xi'an Jiaotong University, China
3Department of Oncology, Xi'an Jiaotong University, China
*Corresponding author: Li ZF, Department of General Surgery, the Second Affiliated Hospital, College of Medicine, Xi'an Jiaotong University, 157 Xi Wu Road, Xi'an 710004, Shaanxi Province, PR. China
Received: July 11, 2014; Accepted: July 20, 2014; Published: July 23, 2014
Keywords
Non-alcoholic steatohepatitis; Tamoxifen; Adverse drug reaction; Breast cancer
Clinical Image
A 38-year-old female with estrogen receptor-positive breast cancer came to our hospital for routine evaluation. She had undergone mastectomy 18 months ago and received oral tamoxifen (20 mg/day) as adjuvant endocrine therapy for 17 months. She had no diabetes mellitus, hypertension, hyperlipidemia or HBV/HCV infection history, and no drinking habit. Her body mass index was 22.5, and liver function was normal before tamoxifen therapy. Laboratory tests revealed an elevated alanine aminotransferase (ALT) (72 U/L) and glutamyltransferase (115 U/L) (both normal up to 40 U/L); the cholesterol, triglyceride, and aspartate aminotransferase were within normal range. Unenhanced abdominal computed tomography (CT) scan showed diffused liver parenchymal density reduction and intrahepatic vascular density was relatively high with a liver/spleen ratio of -0.13 (Figure 1A, 1B). A diagnosis of tamoxifenassociated severe non-alcoholic steatohepatitis (NASH) was made, although absence of histopathological confirmation. Tamoxifen was discontinued, and letrozole (2.5mg/day) was prescribed. Her aminotransferase levels returned to normal limits 6 months subsequently. A repeat CT one year later showed hepatic steatosis remission with liver/spleen ratio of 1.0. She continued to receive letrozole and remains well after 12 months of follow-up.
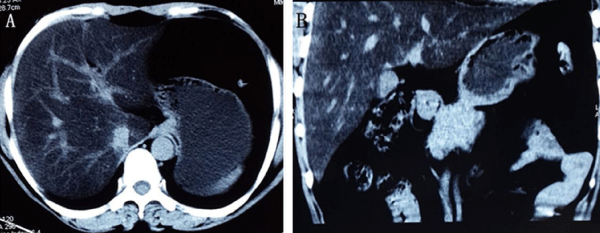
Figure 1: Unenhanced liver computed tomography (CT) scan showed
diffused liver parenchymal density reduction and intrahepatic vascular
density was relatively high with a liver/spleen ratio of -0.13.
Tamoxifen is a nonsteroidal antioestrogen metabolized in the liver. It has been used for decades as adjuvant treatment of oestrogen receptor-positive breast cancer, as well as a chemopreventive agent.1 It is associated with an increased risk of endometrial cancer and other adverse reactions, including the development of hepatic steatosis, with an incidence up to 43% [1-3] The predisposing factors of its occurrence include insulin resistance, obesity, hypertriglyceridemia and CYP17 polymorphism [1-3]. Although hepatic steatosis is readily reversed in most cases end of the therapy [1,2] the disease can progress to cirrhosis [4]. If patients develop severe NASH, alternative therapy includes aromatase inhibitor and/or additional administration with Fibrates. Regular CT examination may be needed to monitor fatty changes of the liver and to differentiate steatosis from metastatic liver tumors during tamoxifen treatment [2].
Financial support: This work was supported by a grant from Program for changjiang Scholars and Innovative Research Team in University (PCSIRT: 1171).
Acknowledgements
This work was supported by a grant from Program for changjiang Scholars and Innovative Research Team in University (PCSIRT: 1171).
References
- Bruno S, Maisonneuve P, Castellana P, Rotmensz N, Rossi S, Maggioni M, et al. Incidence and risk factors for non-alcoholic steatohepatitis: prospective study of 5408 women enrolled in Italian tamoxifen chemoprevention trial. BMJ. 2005; 330: 932.
- Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. Am J Roentgenol. 2003; 180:129-134.
- Ohnishi T, Ogawa Y, Saibara T, Nishioka A, Kariya S, Fukumoto M, et al. CYP17 polymorphism as a risk factor of tamoxifen-induced hepatic steatosis in breast cancer patients. Oncol Rep. 2005; 13:485-489.
- Oien K, Moffat D, Curry GW, Dickson J, Habeshaw T, Mills PR, et al. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999; 353:36-37.