Research Article
J Hepat Res. 2014;1(1): 1004.
Immunofluorescence Detection of Hepatitis B Core Antigen in Formalin Fixed or Frozen Sections of Liver Biopsies from Chronic Hepatitis B Patients
Ruksana Raihan1, Shahina Tabassum1, Mamun A1 Mahtab2*, Afzalun Nessa1, Munira Jahan1, Chowdhury Mohammad Shamim Kabir3, Mohammad Kamal4, Sheikh Mohammad Fazle Akbar5, Julio Cesar Aguilar6
1Department of Virology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
2Department of Hepatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
3Department of Nephrology, National Institute of Kidney Diseases & Urology, Dhaka, Bangladesh
4Department of Pathology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
5Department of Medical Sciences, Toshiba General Hospital, Tokyo, Japan
6Department of Biomedical Research, The Center for Genetic Engineering and Biotechnology, Havana, Cuba
*Corresponding author: Dr. Mamun-Al-Mahtab, Department of Hepatology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
Received: July 02, 2014; Accepted: Aug 05, 2014; Published: Aug 05, 2014
Abstract
Hepatitis B virus (HBV) infection and its liver related complications are a substantial health concern in the Asia-Pacific region. Health interventions and implementation of vaccine programs have substantially reduced the incidence of HBV infections, however, large proportion of individual remains chronically infected and the disease status of the patients has to be evaluated before initiation of antiviral therapy and during follow-up. The immunostaining of HBcAg have proven useful in the characterization of CHB patients from Bangladesh, specifically in HBeAg-negative CHB patients, usually difficult to differentiate from inactive carriers.
The present study was undertaken to detect hepatitis B core antigen (HBcAg) in chronic hepatitis B patients (CHB) and to compare indirect immunofluorescence (IIF) from formalin fixed paraffin block with frozen section of normal saline preparation. Study patients were grouped into HBeAg positive and negative category and HBcAg was detected by using polyclonal rabbit anti HBcAg. Out of 70 study patients 8 (11.4%) had HBeAg positive serology and 62 (88.57%) were HBeAg negative. Among 8 HBeAg positive group all were positive (100%) for core antigen by IIF. Among 62 HBeAg negative patients 55 (88.7%) were positive for core antigen and 7 (11.29%) were negative by IIF. Comparison between frozen section and formalin fixed paraffin block preparation for IIF test for the detection of HBV core antigen from 30 subjects showed, out of 26 HBeAg negative cases 22 (84.62%) were positive for HBcAg by both procedures. Among the 4 HBeAg positive cases all 4 (100%) patients were positive for core antigen by both the procedures.
In conclusion, our results suggest that formalin fixed tissues can be effectively used to detect HBcAg expression in hepatocytes of CHB patients, as compared to the frozen section of liver tissues, providing additional benefits in the case of studying previously analyzed biopsies and considering the limitations to obtain such biological samples.
Keyword: Chronic Hepatitis B Infection; Core Antigen; Indirect Immunofluorescence
Introduction
Hepatitis B (HBV) is one of the most common infectious diseases and the major cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma (HCC). It is estimated that approximately 2 billion people have serological evidence of past or present HBV infection; over 350 million people worldwide are infected with HBV and around 1 million die due to its consequences annually [1,2,3,] Of the estimated 50 million new cases of HBV infection diagnosed annually, 5-10% of adults and up to 90% of infants will become chronically infected, 75% of these in Asia, where hepatitis B is the leading cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma [4]. Bangladesh is a densely populated country with intermediate endemicity (2-7%) for chronic hepatitis B (CHB), where the life time risk of acquiring HBV infection is between 20% to 60%5. Various studies from Bangladesh show that HBV is responsible for 31.25% cases of acute hepatitis, 76.3% cases of chronic hepatitis, 61.15% cases of cirrhosis of liver and 33.3% cases of hepatocellular carcinoma [6,7,5]. With a population of 150 million, Bangladesh has a high (7.3-7.5%) HBsAg positivity among adults [8].
CHB infection is defined as when a person is positive for hepatitis B surface antigen (HBsAg) for at least 6 months. HBV infection is associated with three different structural antigens, hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg), and hepatitis B e antigen (HBeAg).
The nucleocapsid antigen can be found in two closely related antigenic forms, the HBcAg and the HBeAg [9]. HBcAg is detectable in the hepatocyte nucleus or cytoplasm, but never in blood [10,11,12]. Presence of HBcAg in hepatocytes is related to the presence of HBeAg as a marker of HBV replication, and usually connected with active inflammation of liver disease [13,14,15]. However, a significant proportion of patients with CHB are infected with a variant form of HBV which decreases or abolishes the production of HBeAg, mostly due to mutations on the pre core or core promoter regions [16,17]. Such variants of infection is called HBeAg negative chronic hepatitis [17,15]. For HBeAg-negative chronic hepatitis B patients with core promoter or pre core mutations, the presence of HBeAg is not an indicator of viral replication and similarly the HBeAg-negative serology doesn't mean absence of disease progression or absence of intracellular HBV replication creating confusion during the characterization of patients before therapy. It has been proposed that the detection of HBcAg in liver tissues is a better indicator of active viral replication than HBeAg and that the distribution pattern of the HBcAg between nucleus and cytoplasm may be indicative of damage and repairing processes. In addition, the immunostaining of HBcAg have proven useful in the characterization of CHB patients from Bangladesh, specifically in HBeAg-negative CHB patients, usually difficult to differentiate from inactive carriers.
The present article is aimed at studying the HBcAg immunodetection in liver biopsy tissue from CHB patients using samples from two different sources: (a) from paraffin embedded - formalin fixed tissues, and (b) from frozen sections of normal saline preparations.
Materials and Methods
A total of 70 serologically diagnosed CHB patients were selected for this study, according to the selection criteria. Relevant facts from the history and laboratory findings were recorded in a standard predesigned questionnaire/data sheet. Informed written consent was obtained and under all aseptic precaution a Tru-cut liver biopsy was done by one specialized hepatologist. About 1 to 1.5 cm of liver tissue was collected in formalin for histological study and immunofluorescence staining. Formalin fixed tissues were transported in test tubes or plastic containers at normal temperature to the laboratory and routine processing with paraffin impregnation was done. Another 1 cm of liver tissue was collected in normal saline, transported in a cold box containing 4 ice packs (temperature was kept at approximately 4°C) and preserved at -20°C until use (not more than 15 days) for indirect immunofluorescence staining. Detection of HBcAg from all 70 samples were done by using polyclonal rabbit anti HBcAg (Dako, USA) as primary antibody and immunofluorescence staining was done by fluorescein isothiocyanate conjugated secondary antibody (polyclonal Swine Anti Rabbit Immunoglobulin/FITC, Dako). Statistical analysis was done by SPSS 16 version.
Results
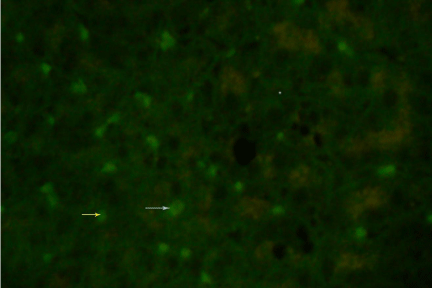
The present study was carried out among 70 serologically diagnosed CHB patients. The study population was grouped into HBeAg positive and negative. CHB patients. The study population was grouped into HBeAg positive and negative. The representative staining patterns of HBcAg expression in negative control (Figure. 1), formalin-fixed liver specimen (Figure. 2.1), and frozen section of liver tissue (Figure. 2.2) have been shown. In addition, the mixed nuclear and cytoplasmic expressions of HBcAg were shown in Figure 3.1 and Figure 3.2. Table 1 show, 8 (11.4%) HBeAg positive and 62 (88.57%) HBeAg-negative patients. Among 8 HBeAg positive group all were positive (100%) for core antigen by IIF from formalin fixed tissue. Among 62 HBeAg negative patients 55 (88.7%) were positive for core antigen by IIF and 7 (11.29%) were negative by IIF from formalin fixed tissue.
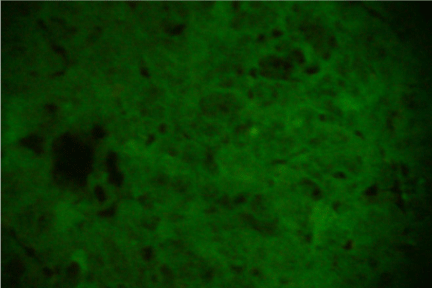
Figure 1: Shows negative control of indirect immunofluorescence test under fluorescence microscope (40X).
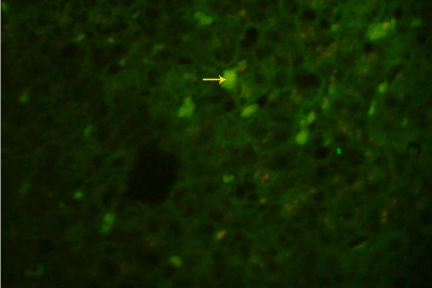
Figure 2.1: Indirect immunofluorescence of HBcAg on formalin fixed liver specimen using polyclonal anti HBcAg (Dako), showing nuclear and cytoplasmic HBcAg expression under fluorescence microscope (40X).
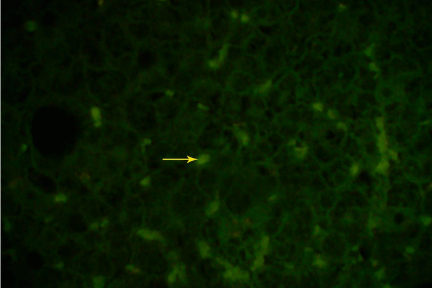
Figure 2.2: Indirect immunofluorescence of HBcAg on frozen section of same (Figute 2.1) liver specimen using polyclonal anti HBcAg (Dako), showing nuclear and cytoplasmic expression of HBcAg under fluorescence microscope (40X).
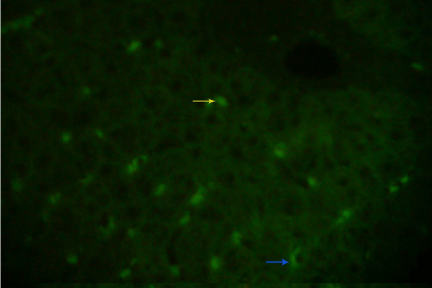
Figure 3.1: Indirect immunofluorescence of HBcAg on frozen section of liver specimen using polyclonal anti HBcAg (Dako), showing a mixed nuclear (yellow arrow) and cytoplasmic (blue arrow) staining under fluorescence microscope (40X).
Figure 3.2: Indirect immunofluorescence of HBcAg on frozen section of same (Figure 3.1) liver specimen using polyclonal anti HBcAg (Dako), showing a mixed nuclear (yellow arrow) and cytoplasmic (white arrow) staining under fluorescence microscope (40X).
HBcAg detection
HBeAg
Total
Positive (n=8)
Negative (n=62)
n = 70
Positive
Negative
8 (100.0) #
55 (88.7)
63 (90.0)
0
7(11.3)
7(10.0)
Total
8 (11.43)
62 (88.57)
70
Table 1: Association of HBV core antigen and HBeAg status among study cases using the formalin fixed - paraffin embedded samples for staining.
Out of 70 cases only in 30 cases, adequate amount of tissues were available for both formalin and normal saline preparation. All 30 subjects were tested for indirect immunofluorescence from both frozen sections and formalin fixed paraffin blocks. Out of 26 HBeAg negative cases 22 (84.62%) were positive for core antigen and 4 (15.38%) were negative for core antigen by both procedures. Among the 4 HBeAg positive cases all 4 (100%) patients were positive for core antigen by both procedures (Table 2).
No of samples
(n = 30)
Frozen section
Formalin fixed paraffin block preparation
HBeAg
HBcAg + ve
HBcAg - ve
HBcAg + ve
HBcAg -ve
Positive
4
4 (100) #
0
4 (100)
0
Negative
26
22 (84.62)
4 (15.38)
22 (84.62)
4 (15.38)
Table 2: Comparison between frozen section and formalin fixed paraffin block preparation of indirect immunofluorescence test for the detection of HBV core antigen from 30 samples.
Discussion
Hepatitis B virus (HBV) infection and its liver related complications are a substantial health concern in the Asia-Pacific region. Over the last two decades public health interventions and implementation of vaccine programs have substantially reduced the incidence of HBV infections. However, large proportion of individual remains chronically infected and the disease status of the patients has to be evaluated before initiation of antiviral therapy and during follow-up [18]. In Bangladesh, 36% of hepatocellular carcinoma is associated with hepatitis B infection [19]. Hepatitis B surface antigen (HBsAg), e antigen (HBeAg), and their respective antibodies (anti-HBs and anti-HBe) have been used as serological markers to determine the status of HBV infection [20]. Serum HBeAg is thought to be associated with active HBV replication and the synthesis of complete virions and has been used to identify highly infectious hepatitis carriers [21,22,23,24]. Other markers of replication include DNA polymerase activity and the hepatitis B core antigen (HBcAg) in hepatocytes [25]. The prevalence of HBeAg-negative variants among HBV patients is quite variable throughout the world, being high in Middle East countries [26]. In case of such variants, detection of HBV core antigen plays an important role along with other markers. Moreover, detection of HBV core antigen can be a better marker than HBeAg for understanding the disease course and for treatment and prognosis [27].
Recent studies throughout the world show HBeAg negativity more than previously suspected. Prevalence of 80 to 90% has been reported from Italy, Greece and in India [28,29,30,31]. In the present study, prevalence of HBeAg negative cases was 88.57% (Table-1). This result indicates a significant increase of HBeAg negative CHB in Bangladesh than similar studies conducted by the Department of Hepatology, BSMMU in 2007, where HBeAg negative prevalence were 51.3% [5].
In the present study, it was observed that all HBeAg positive cases (11.43%) were also positive for HBV core antigen, whereas, among HBeAg negative cases 88.7% were core antigen positive (Table-1). This may be due to higher replication rates of HBeAg positive chronic cases than HBeAg negative chronic cases. A study from Korea also observed a higher prevalence (92%) of HBcAg expression in HBeAg positive patients. On the other hand, for HBeAg negative patients, the 88.7% of HBcAg expression has been the highest level of expression reported for HBcAg staining worldwide. The same Korean study reported 59% positivity of HBcAg detection among HBeAg negative cases [15].
Other studies around the World have shown different levels of HBcAg expression in HBeAg-negative CHB patient. A study from New York showed 48% and another study from Taiwan showed 33% positivity of HBcAg among HBeAg negative cases [32,13].
In this study, IIF was performed from both frozen sections and formalin fixed paraffin block preparations to detect HBV core antigen. Results of both preparations observed similar findings in their detection capacity (Table-2). The frozen section method requires the collection of liver biopsy samples separately in saline tubes which had to be kept frozen at - 20°C until the test was performed (within 15 days of collection). However, the formalin fixed paraffin block preparation did not need separate collection of samples. As such, it was possible to use the same sample collected for histological study for detection of HBcAg also. Moreover, as there is no time limitation for detecting HBV core antigen, if needed, the IIF test may be performed after many years from the preserved paraffin block. Thus, this study observed that it was easier to perform IIF to detect HBV core antigen with the formalin fixed tissues than the frozen section tissues.
In conclusion, our results suggest that formalin fixed tissues can be effectively used to detect HBcAg expression in hepatocytes of CHB patients, as compared to the frozen section of liver tissues, providing additional benefits in the case of studying previously analyzed biopsies and considering the limitations to obtain such biological samples.
According to the results obtained in the present work, it can be proposed the use of paraffin embedded derived tissues as one of the sources of sample for future validation procedures of the intracellular detection of HBcAg.
Acknowledgement
Bangabandhu Sheikh Mujib Medical University (BSMMU) has sponsored the study. We acknowledge the help extended by all the staff of the Dept. of Virology, BSMMU, Dhaka. We are also grateful to all the CHB patients included in this study.
References
- Mast EE, Alter MJ, Margolis HS. Strategies to prevent and control hepatitis B and C virus infections: a global perspective. Vaccine. 1999; 17: 1730-1733.
- Mehdi SR, Pophali A, Al-Abdul Rahim KA. Prevalence of hepatitis B and C and blood donors. Saudi Med J. 2000; 21: 942-944.
- Teresa L Wright M.D. Introduction to Chronic Hepatitis B Infection. Am J Gastroenterol 2006: 101: S1-S6.
- Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000; 15: 1356-1361.
- Mamun-Al-Mahtab, Kumar SI, Rahman S, Kamal M, Khan M, Aggarwal R. Hepatitis B genotypes virus among chronically infected patients in a tertiary-care hospital in Bangladesh. Indian J Gastroenterol. 2006; 25: 219-221.
- Khan M, Zaki KM, Ahmed KU, Ali SM, Islam N. Clinical profile: prognostic index in hepatocellular carcinoma. Bangladesh Med Res Counc Bull. 1991; 17: 49-62.
- Afroz S, Mahtab MA, Rahman S, Khan M. Hepatitis B virus is the leading cause of cirrhosis of liver in Bangladesh. Hepatol Int 2007; 1: 120.
- Alam S, Ahmad N, Mustafa G, Alam K, Khan M. Characteristics of treatment naïve chronic hepatitis B in Bangladesh: younger populations are more affected; HBeAg-negatives are more advanced. Saudi J Gastroenterol. 2008; 14: 15-19.
- Tiollais P, Pourcel C, Dejean A. The hepatitis B virus. Nature. 1985; 317: 489-495.
- Gudat F, Bianchi L, Sonnabend W, Thiel G, Aenishaenslin W, Stalder GA. Pattern of core and surface expression in liver tissue reflects state of specific immune response in hepatitis B. Lab Invest. 1975; 32: 1-9.
- Chu CM, Liaw YF. Intrahepatic distribution of hepatitis B surface and core antigens in chronic hepatitis B virus infection: hepatocyte with cytoplasmic/ membranous hepatitis B core antigen as a possible target for immune hepatocytolysis. J Gastroenterol 1987; 92: 220-225.
- Chu CM, Liaw YF. Immunohistological study of intrahepatic expression of hepatitis B core and E antigens in chronic type B hepatitis. J Clin Pathol. 1992; 45: 791-795.
- Hadziyannis SJ, Lieberman HM, Karvountzis GG, Shafniz DA. Analysis of liver disease, nuclear HBcAg, viral replication, and hepatitis B virus DNA in liver and serum HBeAg Vs anti HBe positive carriers of hepatitis B virus. Hepatology 1983; 3: 656-62.
- Chu CM, Yeh CT, Sheen I-S, Liaw Y F. Subcellular Localization of Hepatitis B Core Antigen in Relation to Hepatocyte Regeneration in Chronic Hepatitis B. J Gastroenterol 1995; 109: 1926-1932.
- Kim TH, Cho EY, Oh HJ, Choi CS, Kim JW, Moon HB. The degrees of hepatocyte cytoplasmic expression of hepatitis B core antigen correlate with histologic activity of liver disease in the young patients with chronic hepatitis B infection. J Korean Med Sci. 2006; 21: 279-283.
- Funk ML, Rosenberg DM, Lok AS. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. J Viral Hepat. 2002; 9: 52-61.
- Hou J, Liu Z, Gu F. Epidemiology and Prevention of Hepatitis B Virus Infection. Int J Med Sci. 2005; 2: 50-57.
- Khan M, Ahmed N. Seroepidemiology of HBV and HCV in Bangladesh. Intern Hepatol Commun 1996; 5: 27-9.
- Pao CC, Yao DS, Lin CY, Kao SM, Tsao KC, Sun CF. Serum hepatitis B virus DNA in hepatitis B virus seropositive and seronegative patients with normal liver function. Am J Clin Pathol. 1991; 95: 591-596.
- Govindarajan S, Fong TL, Valinluck B, Edwards V, Redeker AG. Markers of viral replication in patients with chronic hepatitis B virus infection. Am J Clin Pathol. 1988; 89: 233-237.
- Scott JS, Pan PE, Pace RA, Sloots TP, Cooksley WG. The absence of hepatitis B virus DNA in hepatitis B e antigen positive sera from chronic hepatitis B surface antigen carriers in China. J Med Virol. 1990; 30: 103-106.
- Maruyama T, Iino S, Koike K, Yasuda K, Milich DR. Serology of acute exacerbation in chronic hepatitis B virus infection. Gastroenterology. 1993; 105: 1141-1151.
- van Ditzhuijsen TJ, Selten GC, van Loon AM, Wolters G, Matthyssen L, Yap SH. Detection of hepatitis B virus DNA in serum and relation with the IgM class anti-HBc titers in hepatitis B virus infection. J Med Virol. 1985; 15: 49-56.
- Borg F, Kate FJW, Cuypers HTM, Kuijper AL, Oosting J, Dillen PME, et al. Relation between laboratory test results and histological hepatitis activity in individuals positive for hepatitis B surface antigen and antibodies to hepatitis B e antigen. Lancet 1998; 351: 1914-8.
- Uzun Y, Bozkaya H, Erden E, Cinar K, Idilman R, Yurdaydin C. Hepatitis B core antigen expression pattern reflects the response to anti-viral treatment. J Gastroenterol Hepatol. 2006; 21: 977-981.
- Lampertico P, Del Ninno E, Manzin A, Donato MF, Rumi MG, Lunghi G. A randomized, controlled trial of a 24-month course of interferon alfa 2b in patients with chronic hepatitis B who had hepatitis B virus DNA without hepatitis B e antigen in serum. Hepatology. 1997; 26: 1621-1625.
- Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001; 34: 617-624.
- Shiha G, Samir W, Zalata K, Seif S, Moanis A, Gabere M, et al. Characterization of incidentally detected asymptomatic hepatitis B positive subjects in Egypt. Arab J Gastroenterol 2007; 8: 74-79.
- Dixit VK, Panda K, Babu AV, Kate MP, Mohapatra A, Vashistha P. Asymptomatic chronic hepatitis B virus infection in northern India. Indian J Gastroenterol. 2007; 26: 159-161.
- Huang YH, Hung HH, Chan CC, Lai CR, Chu CJ, Huo TI. Core antigen expression is associated with hepatic necroinflammation in e antigen-negative chronic hepatitis B patients with low DNA loads. Clin Vaccine Immunol. 2010; 17: 1048-1053.