
Letter to the Editor
J Hepat Res. 2014;1(2): 1008.
The Contact Activation of Hepatic Stellate Cells, a New Hypothesis
Chunmeng Jiang*, Xuan Du and Xiaoyun Gao
Department of Gastroenterology, Dalian Medical University, China
*Corresponding author: Chunmeng Jiang, The Second Hospital of Dalian Medical University, Dalian China
Received: August 11, 2014; Accepted: August 20, 2014; Published: August 21, 2014
The activation of Hepatical Stellate Cell (HSC) is the key mechanism of liver cirrhosis. Cytokines are regarded as the main mechanism which leads to the activation of HSC. Many researches in recent decals show that Platelet-Derived Growth Factor (PDGF) Transforming Growth Factor-β1 (TGF-β1) Vascular Endothelial Growth Factor (VEGF) and many other cytokines play an important role to trigger HSC activation, and more and more de nova cytokines such as leptin and chemo tactic factor and so on are involved in the process. However, the treatment remedy derived from these mechanisms show some effects in vitro, but greatly reduced back in vivo. The results about the activation of HSC are entangled more and more cytokines with seldom effect for the treatment, which allows us to consider whether there are more complex mechanisms leading to the activation of HSC which beyond the cytokines. Our recent result showed a quilt different pathway for the activation of HSC as a contact activation manner.
By using of liver biopsy from chronic hepatitis B patients with liver fibrosis, we stained the HSC specific antigen (Desmin), and found that with the progression of fibrosis the HSC showed a chain arrangement. Whether this unique distribution of HSC can prompt cell contact and play an important role in the activation of HSC?
In this study, HE stain, immunofluorescence stains for HSC specific antigen (Desmin), and DAPI were performed. The results showed that within the normal liver tissue, the distribution of quiescent HSC diffused in the liver; when the local inflammation and necrosis of liver appeared, the HSC surrounding necrotic foci present unique banded arrangement, the HSCs nearby was significantly less than those in normal tissue. This banded arrangement raise a question that, when deduce by the cytokines activated theory, the HSC should be arranged according to the concentration gradient of cytokines by a diffused manner, the activated HSC should be radial arrangement, instead of banded arrangement. Although the explanation for this phenomenon is that activated HSC can be chemo tactic movement to the nidus just like fibroblasts, but this can't explain why HSC were banded arrangement. The premise of HSC migration is based on tissue injury, however liver lobule structure which the fiber septums cross over is integrity, there is no obvious injury. Even by HSC migration, it should also be focused on injury center, diffuse outward radial expansion, instead of breakthrough hepatic lobules and formed a strip fibrous septum by a definite direction. Therefore HSC migration theory cannot explain why the HSC distribute like a chain. As the progressing of fibrosis, there almost no HSC in normal hepatic sinusoid. The HSC migrated one by one from inflammatory region to non inflammatory region, and forming a banded arrangement like a chain (Figure 1). We can see from the Figure 2 that there is no fibrous septum formation on the left side of HSC inflammatory foci. But the banded arranged HSC on the right side of inflammatory foci has formed a fibrous septum. This illustrate that the HSC cell chain or ribbon distribution comes first, the formation of fibrous septum comes second, rather than the theory that most of HSC were chemo tactic to the fibrous septum after the fibrous pectum formed. It also confirmed the suspicion above. When the courses arrive to the fibrosis stage, the majority of HSC are distributed in the fibrous septum, which present as a head to tail connected and distribute as a "chain-like" special arrangement. The connection between the cells was visible. Observe from morphology there are also significant differences between these cells. We know the shape of HSC will change largely after it activated, such as the cell body enlarge, cell epitomes increase and extend, gain of cytoskeleton protein. So we can infer that the degree of activation of each HSC arranged in the chain in the picture was significantly different. HSC in the upper part of the chain changed their morphology significantly when compared with HSC in the lower part of the chain which remain irregular shape (means at a quiescent or low activation status) (Figure 2).
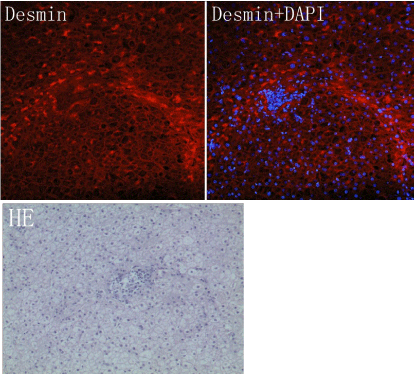
Figure 1: The HE stain show a focal necrosis, there is no obvious fibrous spectrum, while in immunofluorescence stain of Desmin, the HSC had been showed arranging in a chain manner before the fibrotic space formation.
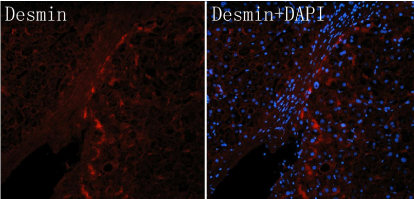
Figure 2: The chain of HSC in a fibrous spectrum, which showing a different morphology, the cells in the upper part of the chain in a active manner, while the lower part showing a quiescent manner.
According to this phenomenon, we propose an adventurous hypothesis: it is the active HSC which accelerates the activation of the adjacent or migrating HSC after they contact, and by the same way they activated the post HSC, and then form the line of activated HSC like a "link", rather than the diffuse form of cytokines release, thereby forming a fiber spacing. Thus the connection between the cells may have an important effect on the activation of HSC.
Conclusion
The activated HSC may lose the contact inhibition characteristic, and transfer into the contact activation; this might be an important mechanism for the activation of HSC.