
Research Article
J Hepat Res. 2014;1(3): 1012.
Hepatic Steatosis And Necro-Inflammato ry Activity Overestimate Liver Stiffness by Transient Elastography in Staging Liver Fibrosis in Chronic Hepatitis C
Luca Rinaldi1, Luciano Restivo1, Barbara Guerrera1, Rosa Zampino1, Aldo Marrone1, Giuseppe Pasquale2, Rosario Zappalá3, Riccardo Nevola1 and Luigi Elio Adinolfi1*
1Department of Medical, Surgical, Neurological, Metabolic, and Geriatric Sciences, Second University of Naples, Italy
2Department of Infectious Diseases, Second University of Naples, Italy
3Evangelic Hospital "Villa Betania", Ponticelli, Naples, Italy
*Corresponding author: ToshLuigi E Adinolfi, Department of Internal Medicine, Clinical Hospital of Marcianise, Second University of Naples, 80125 Marcianise (CE), Italy
Received: August 07, 2014; Accepted: September 10, 2014; Published: September 11, 2014
Abstract
Introduction: Transient Elastography (TE) is a non-invasive method to evaluate liver fibrosis by measuring Liver Stiffness (LS). However, its role in the full management of chronic hepatitis C patients is not completely appraised as well as its limitations are scantly explored. In particular the impact of liver steatosis and necro-inflammatory activity require being more investigated. Thus, this study was aimed to further assess the reliability of TE in evaluating liver fibrosis and the impact of hepatic necro-inflammatory activity and steatosis on the performance of TE.
Patients and Methods: Enrolled were 258 consecutive patients with chronic hepatitis C who underwent to liver biopsy. Hepatic fibrosis was scored according to METAVIR, steatosis and necro-inflammatory activity were also scored. LS ranges were defined according to Castéra. Concordance between liver biopsy and TE was evaluated by Kappa index test. The performance of TE was assessed by ROC curves and by calculating AUROC. Factors independently associated with LS were weight up by logistic regression analysis.
Results: The data showed a high diagnostic accuracy of TE for severe fibrosis (≥F3) with an AUROC of 0.80 and 0.95 for F3 and F4, respectively, with a high specificity and sensitivity; but a lower efficiency in discriminate F1 from F2. At univariate analysis TE showed a relationship with liver fibrosis (p<0.0001),liver inflammation (p<0.0001) and steatosis (p<0.006).Overall, multivariate analysis showed that factors independently associate with LS were liver fibrosis (p<0.0001) and inflammation (p<0.005), whereas, steatosis (p<0.005) was independently associated with LS in patients with fibrosis lower then F3.
Conclusion: Our study confirms that TE is a reliable tool to individuate chronic hepatitis C patients with advanced liver fibrosis or cirrhosis, but it has lesser accuracy for earlier stages of liver fibrosis. Furthermore, high levels of liver necro-inflammatory activity overestimate LS and steatosis induces misevaluation of LS by TE in non-cirrhotic patients.
Keywords: Transient elastography; Chronic hepatitis C; Steatosis; Necro-inflammatory activity; Fibrosis stages; Liver stiffness
Introduction
Hepatic fibrosis is defined as the excessive accumulation of extracellular matrix proteins, resulting from chronic liver inflammatory insults [1]. Hepatic fibrosis is a key determinant of morbidity and mortality in the natural history of chronic hepatitis C in which liver cirrhosis and its complications are the final stage of the disease. Fibrosis assessment is useful to determine the prognosis of the disease, to establish the optimal timing for therapy, screening and surveillance strategies and to predict the response to treatment [2-4]. In particular, identification of patients with cirrhosis is crucial for both to start screening and treatment. Guidelines have defined two stages of liver fibrosis that notably modify the management of HCV patients in clinical practice [5] defining a significant fibrosis a stage greater than F2 according to METAVIR [6].Thus, an accurate evaluation of liver fibrosis in chronic hepatitis C has a pivotal role in its overall management. Liver biopsy is considered the best available approach to evaluate the global burden of the hepatic disease. In fact, liver biopsy is not only the gold standard in assessing liver fibrosis in chronic hepatitis C, but it is able to confirm the aetiology and to assess other significant hepatic alterations (i.e. hepatic steatosis, hepatocellular iron overload and necro-inflammatory activity). However, liver biopsy is often limited by its invasiveness and rare, but serious, complications, including bleeding, pneumothorax, and procedure-related death [6,7]. In addition, it has been reported a percentage of diagnostic inaccuracy due to sampling variability [8,9] and thus, the necessity to have an adequate sampling, which has been set up at least to 20 mm in length and 11 portal spaces [10].
In the last few years, transient elastography (TE; Fibro Scan®, EchoSens, Paris, France) has emerged as a useful, rapid and reproducible tool to measure Liver Stiffness (LS) as an accurate marker to predict liver fibrosis degree [11-13]. Overall, many studies confirmed that TE has good accuracy to diagnose advanced fibrosis and cirrhosis [14,15], although, debate remain on its full diagnostic potential in staging liver fibrosis as well as its reproducibility using the proposed cut off. In addition, TE has a number of limitations, in fact LS may be influenced by acute liver injury (as reflected by ALT flares) with a risk of overestimating LS [16,17], and also by extra hepatic cholestasis [18], irrespective of fibrosis; moreover, TE is difficult to perform in patients who are obese or who have narrow intercostals spaces [19]. It has also been reported that liver necro-inflammatory may be a factor that influence LS [20]. Additionally, it is not clear if steatosis affect the correlation between LS and fibrosis stage. Recent studies on the matter showed that steatosis induces misevaluation of LS [21,22]. However, the real impact of steatosis on LS remains debateable, because in some other studies, steatosis did not have a significant influence on LS [12]. Therefore, considering the high prevalence of steatosis and its preeminent role in both progression and management of chronic hepatitis C infection [23], additional studies are necessary to clarify whether steatosis can influence LS. For all the above considerations, it is obvious that further studies are necessary to pinpoint the full diagnostic potential as well as the limitations of TE in the diagnostic management of chronic hepatitis C patients.
Accordingly, our study was aimed to further assess the reliability of TE in evaluating liver fibrosis as well as its limitations and, in particular, the impact of liver necro-inflammatory activity and steatosis on the diagnostic performance of TE in a large series of chronic hepatitis C patients who underwent a liver biopsy.
Patients and Methods
Treatment naïve Italian consecutive patients admitted to our clinic from 2008 to 2012 for chronic hepatitis C diagnostic work-up and underwent to liver biopsy were enrolled. The inclusion criteria were: presence of HCV RNA in serum, increased ALT levels, and no previous antiviral treatment. Moreover, a patient was enrolled if liver biopsy specimen was at least 20 mm length and there were at least 11 portal spaces evaluable. Patients with decompensate cirrhosis, those who refused or had contraindication for liver biopsy, other associated causes of liver diseases, such as HBV and/or HIV positive, alcohol use ≥30g/die, autoimmune, genetic, and drug abusers were excluded.
Serum HCV-RNA positivity was evaluated by PCR HCV (Amplicor, Roche Diagnostics); viral load was evaluated by real time PCR (Monitor HCV-Amplicor; Roche Diagnostics).
At the time of liver biopsy, enrolled patients underwent to routinely blood tests for a complete liver, haematological and renal function; transientelastography was done during a period of 0-2 days prior the liver biopsy.
Local ethics committees approved the study and all patients provided consent prior to be enrolled in the study.
Histological assessment
Percutaneous echography-assisted liver biopsy was performed using the Menghini technique with an 18 G diameter needle. Biopsy samples were fixed in formalin, paraffin embedded, and stained with haematoxylin-eosin and Masson's trichrome. Biopsy specimens were analysed by an expert Pathologist (GP) in a blinded manner of the results of TE. Liver fibrosis was scored according to METAVIR [6] (F0 = no fibrosis; F1, portal fibrosis without septa; F2, portal fibrosis and a few septa; F3, numerous septa without cirrhosis; and F4, cirrhosis), and necro-Inflammatory Activity (HAI) was scored according to Knodell [24]. Steatosis was scored according to percentage of fat hepatocytes, as follows: 0, < 5%; 1, steatosis in >5-30% of hepatocytes; 2, steatosis in 31-60% of hepatocytes; 3, steatosis >60% of hepatocytes.
Transient elastography
An expert physician (RZ), in the field of echography and elastography, blinded to patient's clinical and biological data, assessed liver stiffness. The measurements were performed on the right lobe of the liver through the intercostals spaces, with the patient lying in the decubitus dorsal position, with the right hand under the head and the head turned toward the left. The tip of the probe was covered with coupling gel and placed on the skin between the ribs at the level of the right lobe of the liver. According to manufacturer, measurements were considered valid if the following three criteria were satisfactory: (1) if there was at least 10 valid shots; (2) the success rate was at least 60%; and (3) interquartile range was less than 30% of the median LSM value (IQR/LSM -30%) [25]. Ranges of stiffness related to fibrosis stages were defined according to Castera et al [11] as follow: F1: 2.5- 7.0 kPa; F2: 7.1-9.5 kPa; F3: 9.6-12.5 kPa; F4: >12.5 kPa.
Statistical analysis
Data are expressed as means ± standard deviation or median and range according with data distribution. The overall concordances as well as the concordance for each previous and subsequent score of fibrosis (e.g.F2 vs F1 and F3) between staging of liver fibrosis by liver biopsy and TE were evaluated by Kappa index test. Spearman's correlation coefficient was used to individuate variable associated with LS. All variables that were significant associated with LS were evaluated in a model of univariate analysis of variance. Logistic regression analysis was used to individuate the independent variables associated with LS. In particular, the influence of liver necro-inflammatory activity and steatosis on the performance of TE was evaluated. The diagnostic performance of TE for each score of liver fibrosis was evaluated by using Receiver-Operating Characteristic (ROC) curves and by calculating the area under the ROC curve (AUROC). A p<0.05 was assumed to denote significance. All statistical analyses were performed using SPSS software, version 13.5.
Results
Enrolled in the study were 258 out the 265 consecutive liver biopsy proven chronic hepatitis C patients, who fulfil the inclusion criteria. Table 1 shows the characteristics of the patients enrolled. The main data showed that the median age was 50 years old and male was 51%. Obesity (BMI > 30) was present in a small proportion of patients (6.2%). A large proportion of patients (65%) showed to be infected by HCV-genotype 1. Steatosis was observed in 57% of patients. HAI median was 5.0 and liver cirrhosis was observed in 14% of patients.
Age, median (range)
50 (20-64)
Male sex
51.40%
BMI
25.2 ± 3.2
Past Drug users
8.10%
Alcohol use (<30 g per day)
5.80%
Blood transfusion
12.40%
HCV RNA, UI/ml; median x 105 (range)
5.4 ( 0.1-2400)
HCV genotype:
1
65.10%
2
25.90%
3
9.00%
Serum ALT, UI/mL (mean±s.d.)
112 ± 87
Serum g-GT, UI/mL (mean±s.d.)
58 ± 46
Liver histology:
HAI score, median (range)
5.0 (1-18)
Fibrosis, score, median (range):
F0
3.90%
F1
36.80%
F2
22.80%
F3
22.00%
F4
14.50%
Steatosis
57%
Table 1: General characteristics of the 258 chronic hepatitis C patients.
Table 2 reports the distribution of patients accordingly with histological liver fibrosis score and LS. Analysis of the data show that an overlapping between fibrosis score 1, 2 and 3 occurred when evaluated by TE (2.5-12.5 kPa); in particular, 25% of patients with histological finding of fibrosis F1 had a stiffness that considered such patients as F2; on the other hand there were 26% of patients with histological finding of F2 who were classified as F1 and 16.9% were classified as F3 at LS. The Kappa index showed an overall good concordance between LS and fibrosis at liver biopsy (Kappa = 0.64). However, an evaluation the Kappa index among F1-F3 stages showed a good concordance between LS and liver histology with a range of Kappa of 0.63 -0.70. Whereas when Kappa index was evaluated for F4 against all other stages of fibrosis an excellent concordance was observed with a Kappa ranging from 0.93 to 0.95.
Metavir Score/F0-F1 F2 F3 F4
KiloPascal (range)° 2, 5-7, 07, 1-9, 5 9, 6-12, 5 >12, 5
Histological score°
F0 (n=9)
9 (100%)
Steatosis (score)
44.4% (1.0)
F1 (n=96)
71 (73.9%) 25 (26.1%)
Steatosis (score)
49.3% (1.3) 64% (1.6)*
F2 (n=59)
11(18.6%) 38 (64.5%)10 (16.9%)
Steatosis (score)
54.5% (1.2)52.6% (1.25) 80%(1.75)*
F3 (n=57)
5(8.8%) 40 (70.1%) 12 (21.1%)
Steatosis (score)
60% (1.66) 55% (1.45) 58.3% (1.71)
F4 (n=37)
3 (8.1%) 34 (91.9%)
Steatosis (score)
66.6% (1.5) 70.6% (1.5)
Table 2: Distribution of hepatic fibrosis and steatosis according to liver stiffness and histologyin the 258 patients with chronic hepatitis C.
The high diagnostic performance of TE in classify patients with cirrhosis (F4) or F3 was also showed by the ROC curve with an AUROC of 0.95 and 0.80 for F4 and F3, respectively (Figure 1), with a sensitivity and specificity of 80% and 85%, respectively for F3, and 85% and 94%, respectively for F4 and the positive and negative predictor values were 80% and 91%, respectively for F3, and 81% and 97%, respectively for F4. The ROC curve confirms the lower efficiency of TE in discriminate F1 from F2 and vice versa with an AUROC of 0, 74 (data not showed in Figure).
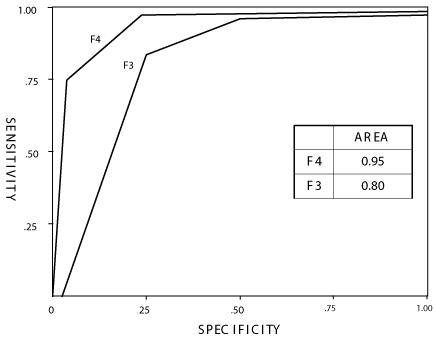
Figure 1: ROC curves including all data of liver stiffness for severe liver fibrosis (METAVIR F3 and F4). Area = AUC. Sensitivity and Specificity for F3 were 80% and 85% and for F4 were 85% and 94%, respectively. For positive and negative predictive values see results in the text. AUC values for F0-F2 are reported in the text, results section.
In Table 2 are also reported the prevalence and scores of liver steatosis accordingly with values of LS. A significant increase (p<0.0001) of both prevalence and score of steatosis was observed in the group of patients with F1 and F2 and LS higher than that expected on the basis of liver biopsy.
An analysis of the factors associated with liver stiffness is reported in Table 3. The overall data showed that a significant relationship was found between LS and histological fibrosis (p<0.0001), HAI (p<0.0001), steatosis (p<0.006) and ALT (p<0.05). BMI was not significant associated with liver stiffness. In addition, a strict correlation was observed between HAI and steatosis, ALT and fibrosis (data not showed).
Score
r
p<
Liver Fibrosis
0.773
0.0001
Liver HAI
0.541
0.0001
Liver Steatosis
0.294
0.006
ALT
0.212
0.05
Table 3: Sperman's correlation of factors* significantly associated with liver stiffness.
The logistic regression analysis including all factors positively associated at univariate analysis to individuate factors independently associate with liver stiffness is reported in Table 4. The multivariate analysis including all patients showed that the independent factors associated with stiffness were liver fibrosis (p<0.0001) and HAI (p<0.005). However, when analysis was done including only patients with liver fibrosis <F3, in addition to fibrosis and HAI, an independent factor associate to LS was steatosis (β 0,038 ± 0, 34; 95% C.I, 0.001-0, 08; p<0.05).
Variable β± S.D.
95% C.I.
p<
Liver Fibrosis
0,705 ± 0, 0710,56- 0,85
0,0001
Liver HAI
0, 046 ± 0, 230,001 - 0,09
0,005
Table 4: Logistic regression analysis of factors independently associated with liver stiffnessin the overall population.
Discussion
The results of this study confirm that TE is a reliable non-invasive means to evaluate liver fibrosis in chronic hepatitis C patients with an overall good concordance with liver biopsy (kappa index 0.64).
However, a substantial overlapping of liver stiffness values was observed between contiguous stages of hepatic fibrosis, particularly for lower fibrosis stages (F1 and F2). Thus, in such stages the lowest diagnostic performance of TE was observed, although, always, in a good concordance with liver biopsy (Kappa 0.66). On the other hand, TE demonstrated to have an excellent capacity to identify patients without liver fibrosis (F0).In concert with other reports [11,12], our data showed that TE had a high diagnostic performance to detect liver cirrhosis with an outstanding concordance with liver biopsy (kappa index 0.93). The ROC curve showed an AUROC of 0.95 for F4 stage confirming that TE has a high accuracy to diagnosis liver cirrhosis. Moreover, our data showed that TE also had a good accuracy in detecting severe fibrosis (F3) with a Kappa index of 0.70 and an AUROC of 0,80.These data are in agreement with earlier reports from Western nations [11,12,14,21,26-28]. In Table 5 are illustrated such previous data showing that TE has an excellent performance in detecting liver cirrhosis (F4). Furthermore, comparative studies in cirrhotic patients have demonstrated that TE has a higher diagnostic performance of other non-invasive tests that showed lower AUROC, e.g. 82% with platelet count, 80% with Fibro Test, 78% with prothrombin index, 76% with prothrombin time, and 70% with APRI index [11,29].
Author [ref.]
Year
Stage
N° of patients
PPV
NVP
AUC
Ziol [9]
2005
Fibrosis
203
88
56
0,79
Cirrhosis
48
78
97
0,97
Castéra [8]
2005
Fibrosis
137
0,83
Cirrhosis
46
0,95
Ganne-Carrie[23]
2006
Cirrhosis
165
74
96
0,95
Kettaneh [11]
2007
Fibrosis
788
0,79
Cirrhosis
147
0,91
Kamphues [24]
2010
Fibrosis
85
0,9
0,5
0,81
Cirrhosis
9
0,9
0,5
0,87
LupsorPlaton [19]
2013
Fibrosis
771
90
70
0,88
Cirrhosis
374
86
97
0,97
Ferraioli [25]
2013
Fibrosis
65
87
74
0,86
Cirrhosis
32
78
96
0,97
Table 5: Literature data on diagnostic accuracy of Fibro scan in detecting liver fibrosis or cirrhosis in HCV patients.
Our study adds another piece to the complex puzzle that must be completed before accepting extensively the use of TE in clinical practice. Our data further support and reinforce the previous proposal that TE can be used in clinical practice as a valid tool in the diagnosis of advanced liver disease in patients with chronic HCV infection. Although our study clearly shows that TE is a valid means in individuate chronic hepatitis C patients with advanced liver fibrosis, some limitations must be kept in mind before assuming the validity of the result in a single case. As also showed by others authors [20], the data of our study indicate that liver necro-inflammatory is an independent factor that may influence liver stiffness evaluation, thus in patients that likely have high HAI, TE should be interpreted with caution because LS may overestimated liver fibrosis. HAI is associated with higher levels of ALT, so in clinical practice high serum ALT levels could be a surrogate of higher HAI and thus in patients with high serum ALT levels TE measurements should be carefulness interpreted. In our study, at univariate but not at multivariate analysis, ALT levels were associated with LS. LupsorPlaton et al. [22] showed that LS correlated significantly with ALT levels. The possibility of overestimating LS values has also been reported for ALT flares in patients with acute viral hepatitis [16] during cholestasis [18] or congestive heart failure [30]. Thus, the interpretation of LS in patients with high ALT levels should be careful evaluated. It has been suggested that if ALT levels are 3 times the normal value, there is a risk of overestimating the fibrosis stage [31].
Our data show that higher levels of steatosis were observed in patients with LS higher than that expected on the basis of results of liver biopsy; in addition steatosis was correlated with stiffness at univariate analysis; moreover, when multivariate analysis was done including only patients with liver fibrosis less thanF3, steatosis was an independent factors associated with higher values of LS. These data seem to indicate that liver steatosis and in particular high levels, could induce erroneous assessment of liver stiffness particularly in patients with fibrosis score less than F3, whereas steatosis did not seem to influence LS in patients with significant fibrosis (F3) or cirrhosis. Similar results have been reported in a recent accurate and specific study on the matter showed that high level of steatosis induces misevaluation of liver stiffness [21]; moreover, Ziol et al [12] also confirmed that steatosis may affect LS that was negligible in cirrhotic patients but it was significant in non-cirrhotic patients; Lupsor Platon M. et al.[22] reported that LS was independently influenced by liver steatosis. Thus, TE could be inaccurate to assess liver fibrosis in chronic hepatitis C patients with high levels of steatosis. However, further studies are necessary to clarify how steatosis can influence LS.
It is also important to underline that BMI is associated with liver metabolic steatosis and that overweight/obesity is a limitation for the use of TE [32].Our study did not show any positive or negative association between BMI and LS, perhaps due to the very low number of obese patients include in the study; however, many other studies showed that obesity was an independent factor associated with measurement failure [32,33].
Overall data seem to indicate that HCV genotype do not influence the performance of TE in assessing liver fibrosis. However, considering the significant differences between HCV genotypes in term of biological, metabolic and therapeutic characteristics, a different utilization and performance of TE could be hypothesized according with HCV genotype. In this respect, the documentation of an advanced stage of fibrosis in genotype 1 and 4 chronic hepatitis C patients is of fundamental importance not only for prognostic value, but also to make a decision on current treatment based on peg-interferon triple regimen. In view of considerable side effects and high cost, such triple association is indicated only for treatment of patients with genotype 1 non-responders to peg-interferon and ribavirinor naive with significant fibrosis [34]. Thus, it is mandatory to individuate patients with significant fibrosis. Considering the good accuracy demonstrated by TE to individuate patients with significant fibrosis, it is possible to suggest that TE is a tool for the right management of such patients. It is important to underline that considering the new approved oral treatment such as sofosbuvir and simeprevir, which have a higher response rate and can be used also without interferon, HCV genotype 1 could no longer be considered as difficult to treat genotype. However, due to the high cost of new treatment, at present, it is not approved in all Country and likely it will be approved with limitations, perhaps based on liver significant levels of fibrosis; in addition, it has been demonstrated that an optimized treatment, particularly for cirrhotic patients, with new oral agents should be done in combination with peg-interferon and ribavirin [35] or in alternative utilizing the combination sofosbuvir plus simeprevir with obvious increased of cost [36,37]. Thus, TE may yet have a role in the era of new oral direct-acting antiviral for hepatitis C.
HCV genotypes 2 and 3 are commonly considered easy-to-treat. To date, according to guidelines, histological definition is not considered necessary for patients infected with HCV genotype 2 or 3, except for those cases with relative contraindications, not motivated or elderly age [38]. Thus, also TE is not so mandatory as for HCV genotype 1, but can be of great help in define the stage of the disease especially in the above exception. It is important to underline that HCV genotype 3 is associated with high prevalence and levels of hepatic steatosis that could overestimate liver fibrosis if evaluated with TE. It has been showed that the absence of steatosis is a significant predictor of sustained virologic response in patients infected with HCV genotype 2 and 3 [39]. In addition, recently has been demonstrated [40] that hepatic steatosis significantly increases the risk of relapse in patients with genotype 3 who achieve a rapid virologic response with interferon-based regimens. Thus, instead of liver fibrosis, these data indicate that the quantification of steatosis appears as a fundamental element for the management of chronic hepatitis C genotype 3. However, it has been reported that, when approved, new oral treatment and in particular the combination sofosbuvir and ribavirin should be the treatment of choice for genotypes 2 and 3 [35].
Conclusion
TE is a non-invasive method that has been recently introduced for the assessment of hepatic fibrosis in patients with chronic liver diseases, by measuring liver stiffness. The technique is easy to perform, rapid, well tolerated by patients and repeatable, thus allowing a follow-up close in time. Our study confirms that TE isare liable tool to individuate chronic hepatitis C patients with advanced liver fibrosis or cirrhosis, but it has lesser accuracy for earlier stages of liver fibrosis. Furthermore, high levels of liver necro-inflammatory activity and steatosis induce a misevaluation of liver fibrosis by TE. Thus, in order to avoid false results, TE should be done and interpreted by an expert clinician who should evaluate the TE results accordingly to the full clinical and biochemical context. Keeping in mind the above limitations, we believe that TE may be used confidently in clinical practice as an integrated system to allow a safer, efficient and appropriate diagnostic and therapeutic management of chronic hepatitis C patients.
Acknowledgement
The study was supported by a grant from Regione Campania, Italy.
References
- Zampino R, Marrone A, Restivo L, Guerrera B, Sellitto A, Rinaldi L, et al. Chronic HCV infection and inflammation: Clinical impact on hepatic and extra-hepatic manifestations. World J Hepatol. 2013; 5: 528-540.
- Crisan D, Radu C, Dan Grigorescu M, Lupsor M, Feier D, Grigorescu P. Prospective Non-Invasive Follow-up of LiverFibrosis in Patients with Chronic hepatitis C. J GastrointestinLiv Dis. 2012; 21: 375-382.
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003; 38: 38-53.
- Martinez MS, Foucher J, Combis J M, Metivier S, Brunetto M, Capron D, et al. Longitudinal Liver Stiffness Assessment in Patients with Chronic Hepatitis C Undergoing Antiviral Therapy. Ploss One. 2012; 7: 47715.
- National Institutes of Health . National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C. Hepatology. 2002; 36: 3-20.
- Intra-observer and inter-observer variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology.1994; 20: 15-20.
- Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009; 50: 1-3.
- Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002; 97: 2614-2618.
- Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003; 38: 1449-1457.
- Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. American Association for the Study of Liver Diseases . Liver biopsy. Hepatology. 2009; 49: 1017-1044.
- Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005; 128: 343-350.
- Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005; 41: 48-54.
- Afdhal NH. Fibroscan (transient elastography) for the measurement of liver fibrosis. Gastroenterol Hepatol (N Y). 2012; 8: 605-607.
- Kettaneh A, Marcellin P, Douvin C, Poupon R, Ziol M, Beaugrand M, et al. Features associated with success rate and performance of FibroScan measurements for the diagnosis of cirrhosis in HCV patients: a prospective study of 935 patients. J Hepatol. 2007; 46: 628-634.
- Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006; 55: 403-408.
- Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, et al. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008; 47: 380-384.
- Sagir A, Erhardt A, Schmitt M, Häussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008; 47: 592-595.
- Millonig G, Reimann FM, Friedrich S, Fonouni H, Mehrabi A, Büchler MW, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008; 48: 1718-1723.
- Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010; 51: 828-835.
- Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008; 57: 1288-1293.
- Boursier J, ZarskiJ P, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria of liver stiffness evaluation by transient elastography. Multicentric group from ANRS/HC/EP23 FIBROSTAR studies. Hepatology. 2013; 57: 1182-1191.
- LupsorPlaton M, Stefanescu H, Feier D, Maniu A, Badea R. Performance of unidimensional transient elastography in staging chronic hepatitis C. Results from a cohort of 1,202 biopsied patients from one single centre. J Gastrointestin Liver Dis. 2013; 22: 157-166.
- Adinolfi LE, Restivo L, Zampino R, Lonardo A, Loria P. Metabolic alterations and chronic hepatitis C: treatment strategies. Expert Opin Pharmacother. 2011; 12: 2215-2234.
- Desmet VJ. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis [Hepatology 1981;1:431-435]. J Hepatol. 2003; 38: 382-386.
- Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003; 29: 1705-1713.
- Ganne-Carrié N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006; 44: 1511-1517.
- Kamphues C, Lotz K, Röcken C, Berg T, Eurich D, Pratschke J, et al. Chances and limitations of non-invasive tests in the assessment of liver fibrosis in liver transplant patients. Clin Transplant. 2010; 24: 652-659.
- Ferraioli G, Tinelli C, Dal Bello B, Zicchetti M, Lissandrin R, Filice G, et al. Performance of liver stiffness measurement by transientelastography in chronichepatitis. World J Gastrenterol. 2013; 19: 49-55.
- Borsoi Viana MS, Takei K, Collarile Yamaguti DC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann Hepatol. 2009; 8: 26-31.
- Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, et al. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010; 52: 206-210.
- Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007; 14: 360-369.
- Sirli R, Sporea I, Bota S, Jurchis A. Factors influencing reliability of liver stiffness measurements using transient elastography (M-probe)-monocentric experience. Eur J Radiol. 2013; 82: 313-316.
- Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006; 18: 411-412.
- Cammá C, Petta S, Cabibbo G, Ruggeri M, Enea M, Bruno R, et al. Cost-effectiveness of boceprevir or telaprevir for previously treated patients with genotype 1 chronic hepatitis C. J Hepatol. 2013; 59: 658-666.
- Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014; 312: 631-640.
- Sulkowski M, Jacobson IM, Ghalib R, Rodriguez-Torres M, Younussi Z, Cerregidor A, et al. Once-daily simeprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in HCV genotype 1 prior null responders with Metavir F0-2: COSMOS study subgroup analysis. 49th Annual Meeting EASL 2014. J Hepatol. 2014.
- Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet. 2014.
- Zeuzem S, Hultcrantz R, Bourliere M, Goeser T, Marcellin P, Sanchez-Tapias J, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004; 40: 993-999.
- Shah SR, Patel K, Marcellin P, Foster GR, Manns M, Kottilil S, et al. Steatosis is an independent predictor of relapse following rapid virologic response in patients with HCV genotype 3. Clin Gastroenterol Hepatol. 2011; 9: 688-693.
- Restivo L, Zampino R, Guerrera B, Ruggiero L, Adinolfi LE. Steatosis is the predictor of relapse in HCV genotype 3- but not 2-infected patients treated with 12 weeks of pegylated interferon-α-2a plus ribavirin and RVR. J Viral Hepat. 2012; 19: 346-352.