
Research Article
J Hepat Res. 2015;2(1): 1018.
Hepatic Steatosis and Metabolic Syndrome Variables: Overplay on the Progression of Fibrosis among Hepatitis C Mono-Infected Patients and Human Immunodeficiency Virus Co-Infected Patients
Rodríguez-Torres M1,2*, Bräu N3,4, Rios-Bedoya CF1,5, Hallman D1,6, Maldonado I1 and Rodríguez- Orengo JF1,7
1Department of Clinical Research, Fundacion de Investigacion, Puerto Rico, USA
2Ponce School of Medicine, Puerto Rico, USA
3Bronx Veterans Affairs Medical Center, USA
4Division of Infectious Diseases and Liver Diseases, Mount Sinai School of Medicine, USA
5Department of Family Medicine, Michigan State University, USA
6Department of Medicine, UPR School of Medicine, USA
7Department of Biochemistry, UPR School of Medicine, USA
*Corresponding author: Rodríguez-Torres M, Department of Clinical Research, Fundacion de Investigacion,, Ponce School of Medicine, Puerto Rico, Ave. Muñoz Rivera #998, Río Piedras, Puerto Rico 00927, USA
Received: January 13, 2015; Accepted: February 09, 2015; Published: February 11, 2015
Abstract
Background & Aims: Hepatic steatosis has been reported to be associated to increased fibrosis progression, reduced response rates to anti-HCV therapy and antiretroviral therapy for which it has clinical importance. This study was conducted to determine the impact of hepatic steatosis on the severity of liver disease progression in chronic HCV mono-infected and HIV/HCV co-infected patients.
Methods: Retrospective study of patients with chronic HCV or HIV/HCV coinfection, with liver biopsies, at the Fundación de Investigación in Puerto Rico and the Bronx Veterans Affairs Medical Center in New York from 1998 to 2005.
Results: Of the 1212 subjects, 834 (69%) were HCV mono-infected and 378 (31%) were HIV/HCV co-infected. Steatosis was more prevalent in HCV patients than HIV/HCV patients (51.2% vs. 39.7%, p=0.003). Severity of necroinflammatory grade was statistically associated to steatosis in HCV patients (p < 0.001), but not HIV/HCV patients (p=0.620). Severity of fibrosis progression rate was associated to steatosis in HCV patients (p=0.088), but not HIV/HCV patients (p=0.493). Hepatic steatosis increased the chances of fibrosis across all levels in the multivariate analysis (using a partial proportional odds model) in HCV patients, but not HIV/HCV patients.
Conclusion: Hepatic steatosis is more prevalent in HCV mono-infected patients, than in HIV/HCV co-infected patients. Presence of steatosis impacted the progression of liver disease differently; it increased the severity of necroinflammatory grade, the fibrosis progression rate and the chances of fibrosis in HCV mono-infected patients, but not in HIV/HCV co-infected patients.
Keywords: Hepatic steatosis; Co-infected; Metabolic syndrome; Chronic hepatitis; Nonalcoholic fatty liver disease; Fibrosis
Abbreviations
ALT: Alanine Aminotransferase; ART: Antiretroviral Therapy; BMI: Body Mass Index; DM: Diabetes Mellitus; FPR: Fibrosis Progression Rate; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; HDL: High-Density Lipoprotein; HIV: Human Immunodeficiency Virus; HS: Hepatic Steatosis; IR: Insulin Resistance; NAFLD: Nonalcoholic Fatty Liver Disease; NASH: Nonalcoholic Steatohepatitis; NRTI: Nucleoside Reverse Transcriptase Inhibitors
Introduction
Liver disease in patients with HIV has been mostly focused on the co-infection with Hepatitis C Virus (HCV) and Hepatitis B Virus (HBV), with less attention to other diseases such as Nonalcoholic Fatty Liver Disease (NAFLD). NAFLD represents a spectrum of liver diseases characterized mainly by macro vesicular steatosis in the absence of significant alcohol consumption, and is now recognized as a major cause of abnormal liver enzymes [1,2]. Hepatic histology can vary from isolated Hepatic Steatosis (HS) alone to Nonalcoholic Steatohepatitis (NASH) that can then progress to cirrhosis and liver failure [3,4]. HS has been reported in patients with both chronic HCV and HIV/HCV co- infection, with an overall prevalence ranging from 40% to 75% [5], which is greater than expected from the general population [6]. A reported meta-analysis, addressing a total of 1,989 HIV/HCV co-infected patients, found a prevalence of HS at 50.8%, ranging from 23% to 72%. Four of these studies included a total of 1,540 HCV mono-infected patients, finding its prevalence of HS at 48.6%, ranging from 33% to 59%. In this meta-analysis, HIV did not confer an increased risk for HS when compared to HCV monoinfection [7]. Although the exact mechanism for the development of NASH has not been fully elucidated, it appears to be associated to mitochondrial dysfunction [8], as a result of several metabolic abnormalities. The majority of patients with NAFLD have Insulin Resistance (IR) and Dyslipidemia, both of which are associated with other features of the metabolic syndrome such as central obesity, Diabetes Mellitus (DM), and hypertension [9,10]. In HIV/HCV coinfected patients, HS has been found to be associated with increased body mass index [11], diabetes [12], elevated ALT levels, HCV genotype 3 [13], necroinflammation, and fibrosis [5,7]. Also common in chronic HCV and HIV/HCV co- infection is the presence of IR. Although the IR mechanism in liver disease is unknown, it apparently is critical in HS and liver disease progression. A study found IR associated with liver fibrosis/steatosis in HCV mono-infected but not in co-infected patients [14], but another did find IR associated with liver fibrosis in co-infected patients [15].
Since HS is associated with increased fibrosis progression [16,17] its identification has clinical importance. In addition, the presence of HS has been reported to be associated with reduced response rates to anti-HCV therapy [18] and Antiretroviral Therapy (ART) [19, 20], for which it has therapeutic importance as well. To determine the impact of HS on the severity of liver disease progression in HCV mono-infected patients and HIV/HCV co-infected patients, this retrospective study was conducted on subjects who had liver biopsies performed as part of their evaluation for chronic HCV [21].
Patients and Methods
Study population
The present study included consecutive patients with chronic HCV infection or HIV/HCV co-infection, who had liver biopsies performed and were being treated at the Foundation de Investigation in San Juan, Puerto Rico and at the Bronx VA Medical Center in New York from 1998 to 2005. Laboratory and virological data, within two months of the liver biopsy dates, were required for inclusion. The study was performed in accordance to the Declaration of Helsinski, approved by Institutional Review Boards at each center and each patient provided a written informed consent and a data release form to use for clinical investigation purposes.
Variables examined
The personal information included: age, sex, Body Mass Index (BMI), ethnicity (Latino or non-Latino), presence of diabetes, amount of alcohol consumed (g/day) and determination of HCV risk factor (injection drug use, blood transfusion or sex). The laboratory data included: triglycerides level (mg/dL), total cholesterol (mg/ dL), High-Density Lipoprotein (HDL) level (mg/dL), total bilirubin (mg/dL) and Alanine Aminotransferase (ALT) levels (U/L). HCV genotyping and HCV RNA quantification were performed using standard commercial kits.
Liver tissue evaluation
The Percutaneous liver biopsies, performed in standard fashion, were formalin-fixed, paraffin-embedded and stained with hematoxylineosin and Masson’s trichrome. The Ishak Histologic Activity Index [22] was used to assess the degree of inflammation and fibrosis in the tissue sample. The necroinflammatory components were totaled to calculate the activity grade from 0 to 18, and the fibrosis was classified as stage 0 to 6. Steatosis was graded as in the modified Brunt scoring system [23,24], by determining the percent of hepatocytes containing fat; where <5% corresponds to grade 0, 5-33% corresponds to grade 1, 33-66% corresponds to grade 2 and >66% corresponds to grade 3. The Fibrosis Progression Rate [FPR] was determined by calculating the quotient of the liver fibrosis stage divided by the interval of time between the biopsy and the time of HCV infection, and expressed as units/year. To minimize intra-observer bias, all liver biopsies were reviewed by pathologists, with experience in liver tissue, whom were blinded to the clinical information. The liver biopsies from Fundacion de Investigation (69%) were performed by a sole invasive radiologist with 3 CT-guided passes, in separate directions; which yielded an average length of 45 cm using a tricut gauge 18 needle. These liver biopsies were analyzed by the same experienced hepatopathologist. The liver biopsies from the Bronx VA Medical Center were performed by the gastroenterology staff and interpreted by experienced pathologists.
Statistical analysis
Statistical analysis was performed using STATA® (v11) [25]. The data are presented as mean value ± standard deviation. Differences between continuous variables were compared using Analysis of Variance (ANOVA) or t-test, and categorical variables were compared using Fisher’s exact tests. A p value of <0.05 was used to determine statistically significant results. For the fibrosis multivariate analysis, associations were analyzed using a partial proportional odds model, since it was expected that at least one of the variables was not going to meet the proportional odds assumption. The partial proportional model [26,27] estimates the likelihood of being in an upper ordinal category of the response variable, conditional on the explanatory variables, and allows a group of explanatory variables to vary over the response variable categories while keeping the others constant. This model has been used in several clinical studies [28-30] but has been used scarcely in HCV research [31] even though it is particularly appropriate to studies involving fibrosis staging.
Results
Study population
1212 subjects were studied; 834 (69%) HCV mono-infected and 378 (31%) HIV/HCV co-infected. The characteristics of each group are presented in Table 1. HCV genotyping and HCV RNA quantification were only available in 937 subjects (76.9%); 673 mono-infected (80.1%) and 264 co-infected (69.8%). There was a statistically significant difference between the groups in regards to the age, ethnicity, alcohol use, risk factors for HCV, HCV genotype, triglycerides levels, HDL levels, total bilirubin levels and BMI. The group of HCV mono-infected patients was: older, consumed more alcohol, had a higher BMI and predominantly HCV genotype 1. The group of HIV/HCV co-infected patients was: predominantly Latinos, had history of intravenous drug use, had higher triglycerides and total bilirubin levels, and lower HDL levels. The co-infected patients had in general well controlled HIV disease, with HIV RNA mean of 594 copies, (n=365 SD 13.3 ) with 80 patients or 22% with less than 50 copies. The mean CD4 was 455.2cells/ml (SD 272.2 and 23% or 87 had less than 250cells/ml. The mean duration of use of ART in 248 patients with information is 3.25 years (SD 2.15 with 79% or 195 patients with therapy for more than 1 year. Of the risk factors for steatosis, the significantly prevalent variables are: alcohol use in the HCV mono-infected group, elevated triglycerides levels in the HIV/ HCV co-infected group, and the high BMI in both groups, although it was significantly higher in the HCV mono-infected patients. The prevalence of diabetics was relatively low (12.5% and 12.9%) in both groups. Also of relevance, only 48 subjects of 937 (5.1%) were HCV genotype 3, with a higher prevalence in HIV/HCV co-infected patients (9.8%) than in HCV mono-infected individuals (3.3%).
Characteristic
HCV mono-infected
(n=834)
HIV/HCV co-infected
(n=378)
P-value
Age (years ± SD)
49.9 ± 10.5
45.2 ± 8.4
<0.001
Sex (male, %)
632 (75.8%)
297 (78.6%)
0.21
Ethnicity (Latinos, %)
647 (77.6%)
313 (82.8%)
0.023
Alcohol use (g/day)
27.4 ± 58.7
17.6 ± 50.1
0.007
Diabetes
105 (12.6%)
48 (12.7%)
0.926
Risk factors for HCV infection
<0.001
Injection drug use
464 (67.1%)
274 (75.9%)
Blood transfusion
180 (26.0%)
16 (4.4%)
Sex
48 (6.9%)
71 (19.7%)
HCV genotype
<0.001
1
543 (80.7%)
204 (77.3%)
2
96 (14.3%)
23 (8.7%)
3
22 (3.3%)
26 (9.8%)
4
12 (1.8%)
11 (4.2%)
log HCV RNA
5.98 ± 1.10
5.95 ± 1.10
0.664
Triglycerides (mg/dL)
134.7 ± 89.9
190.8 ± 108.8
<0.001
Total cholesterol
169.9 ± 37.4
171.8 ± 45.1
0.486
HDL
47.2 ± 22.8
42.6 ± 20.2
0.024
Total bilirubin (mg/dL)
0.81 ± 0.37
0.94 ± 0.53
<0.001
ALT (U/L)
89.6 ± 74.7
87.3 ± 62.3
0.610
BMI
28.4 ± 5.2
27.0 ± 5.0
0.002
Table 1: Patient Group Characteristics Stratified by HIV condition.
Findings on liver tissue evaluation
The prevalence of the different grades of steatosis in the liver tissues is presented in Figure 1. Steatosis was present in 51.2% of the HCV mono-infected patients, and moderate to severe in 16.2%. In the HIV/HCV co-infected patients, steatosis was only present in 39.7% of the patients, and moderate to severe in 10.7%. This difference was statistically significant (p= 0.003), meaning steatosis was more frequent and severe in the HCV mono-infected patients than in the HIV/HCV co-infected patients.
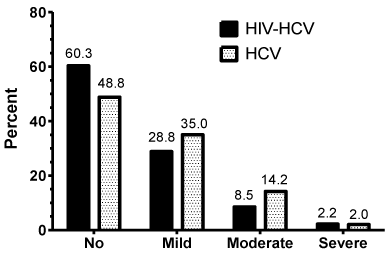
Figure 1: Percentage of HCV mono-infected and HIV/HCV co-infected
patients at different stages of steatosis.
The grading of necroinflammatory features is given using a score from 0 to 18; and the scores in our patients varied from 0 to 15. In HCV mono-infected patients, the distribution of these necroinflammatory scores show a bell-shaped pattern (data not shown) having a higher number of patients with scores of 4 to 8, and peaking at 6. In the HIV/HCV co-infected patients, the distribution of scores (data not shown) has a bi-modal pattern with two mayor peaks, one at the score of 3 and the other at the scores of 6 and 7. Figure 2 presents the mean values of these necroinflammatory scores in association to the different grades of steatosis, where, overall, the highest value is 7.4 and the lowest 5.1. In HCV mono-infected patients the association of these mean values show a positive trend; having a lower value associated with no or mild steatosis and a higher value associated with moderate and severe steatosis. This correlation, in the HCV monoinfected patients, is statistically significant (p<0.001). However, in the HIV/HCV co-infected patients, the correlation of the mean values was not statistically significant (p=0.620).
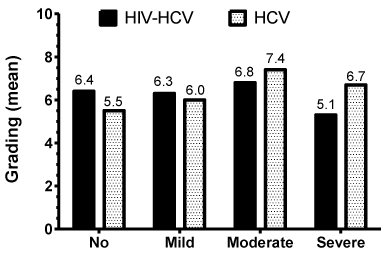
Figure 2: Mean necroinflammatory score at different stages of steatosis for
HCV mono-infected and HIV/HCV co-infected patients.
The fibrosis stage scores range from 0 to 6. In both HCV mono infected and HIV/HCV co-infected patients, the distribution of these scores show a similar irregular pattern (data not shown), resulting 3 as the most frequent score, followed by 1, then 2 and 5 in both groups. When comparing the scores of the HCV mono-infected to those of the co-infected patients, they were not statistically different (p=0.170). Figure 3A presents the mean fibrosis scores in association to the different grades of steatosis, where, overall, the highest value is 3.8 and the lowest 2.1. Since these values are of such small range, and of ordinal nature, the mean values should not be statistically compared. Thus, the data was used for the multivariate analysis using a partial proportional odds model. Table 2 presents the partial proportional odds model with the explanatory variables significantly associated with higher or lower fibrosis stages in HCV mono-infected patients. HS, AST, and age at liver biopsy increase the chances of fibrosis across all levels and met the proportional odds assumption; therefore the magnitude of the relationship across all levels of fibrosis was constant. Note that for HS, there is a significant upward trend for all of its stage over all fibrosis levels. In contrast and although it also met the proportional odds assumption, total cholesterol decreases slightly the chances of fibrosis over all stages. The relationship between fibrosis and Latino ethnicity was not consistent across fibrosis stages. Specifically, Latino ethnicity was significantly related to fibrosis in stages 3 and higher with the strongest positive relationship in stage 6 (stages 6 versus combined stages 0 through 5).
OR (95% CI)
OR (95% CI)
OR (95% CI)
OR (95% CI)
OR (95% CI)
Variables
Ishak Fibrosis
Stage 0 Vs.
Stage 1+2+3+
4+5+6
Ishak Fibrosis
Stage 0+1 Vs.
Stage 2+3+4
+5+6
Ishak Fibrosis
Stage 0+1+2 Vs.
Stage 3+4+5+6
Ishak Fibrosis
Stage 0+1+2+3
Vs. Stage 4+5+6
Ishak Fibrosis
Stage 0+1+2+
3+4 Vs. Stage
5+6
Ishak Fibrosis
Stage 0+1+2+
3+4+5 Vs.
Stage 6
Liver Steatosis
Stage 0
Stage 1
Stage 2
Stage 3
--
1.7 (1.3, 2.4)
3.5 (2.3, 5.4)
4.2 (1.8, 9.9)
--
1.7 (1.3, 2.4)
3.5 (2.3, 5.4)
4.2 (1.8, 9.9)
--
1.7 (1.3, 2.4)
3.5 (2.3, 5.4)
4.2 (1.8, 9.9)
--
1.7 (1.3, 2.4)
3.5 (2.3, 5.4)
4.2 (1.8, 9.9)
--
1.7 (1.3, 2.4)
3.5 (2.3, 5.4)
4.2 (1.8, 9.9)
--
1.7 (1.3, 2.4)
3.5 (2.3, 5.4)
4.2 (1.8, 9.9)
AST
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
Age at Liver Biopsy
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
Total cholesterol
0.99 (0.99, 0.99)
0.99 (0.99, 0.99)
0.99 (0.99, 0.99)
0.99 (0.99, 0.99)
0.99 (0.99, 0.99)
0.99 (0.99, 0.99)
Latino
Yes
0.6 (0.2, 2.0)
1.0 (0.6, 1.5)
1.2 (0.8, 1.7)
2.5 (1.6, 3.9)
2.9 (1.7, 5.0)
3.6 (1.2, 10.5)
Table 2: Adjusted odds ratios for covariates related to Ishak Fibrosis Stage among HCV mono-infected patients.
The results of the partial proportional odds model for HIV/HCV co-infected patients are presented in Table 3. HS, AST, and age at liver biopsy increase the chances of fibrosis across all levels and met the proportional odds assumption; therefore the magnitude of the relationship across all levels of fibrosis was constant. However, in the case of hepatic steatosis, only stage 2 was statistically significant and positively related to all fibrosis stages. The relationships between fibrosis and log HCV RNA levels and Latino ethnicity were inconsistent across fibrosis stages. Specifically, log HCV RNA levels were inversely related to fibrosis stage only in stage 4 and higher, with the biggest decrease (50%) in stage 6 when compared to all other lower stages. Latino ethnicity was significantly related to fibrosis between stages 2 and 4.
OR (95% CI)
OR (95% CI)
OR (95% CI)
OR (95% CI)
OR (95% CI)
OR (95% CI)
Variables
Ishak Fibrosis
Stage 0 Vs.
Stage 1+2+3+
4+5+6
Ishak Fibrosis
Stage 0+1 Vs.
Stage 2+3+4
+5+6
Ishak Fibrosis
Stage 0+1+2 Vs.
Stage 3+4+5+6
Ishak Fibrosis
Stage 0+1+2+3
Vs. Stage 4+5+6
Ishak Fibrosis
Stage 0+1+2+
3+4 Vs. Stage
5+6
Ishak Fibrosis
Stage 0+1+2+
3+4+5 Vs.
Stage 6
Liver Steatosis
Stage 0
Stage 1
Stage 2
Stage 3
--
1.2 (0.7, 1.9)
2.9 (1.2, 6.7)
0.4 (0.1, 1.6)
--
1.2 (0.7, 1.9)
2.9 (1.2, 6.7)
0.4 (0.1, 1.6)
--
1.2 (0.7, 1.9)
2.9 (1.2, 6.7)
0.4 (0.1, 1.6)
--
1.2 (0.7, 1.9)
2.9 (1.2, 6.7)
0.4 (0.1, 1.6)
--
1.2 (0.7, 1.9)
2.9 (1.2, 6.7)
0.4 (0.1, 1.6)
--
1.2 (0.7, 1.9)
2.9 (1.2, 6.7)
0.4 (0.1, 1.6)
AST
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
1.01 (1.01, 1.02)
log HCV RNA
1.3 (0.7, 2.7)
0.8 (0.6, 1.1)
0.9 (0.7, 1.2)
0.9 (0.6, 1.1)
0.6 (0.5, 0.9)
0.5 (0.3, 0.8)
Age at Liver Biopsy
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
1.05 (1.03, 1.07)
Total cholesterol
1.00 (1.00, 1.01)
1.00 (1.00, 1.01)
1.00 (1.00, 1.01)
1.00 (1.00, 1.01)
1.00 (1.00, 1.01)
1.00 (1.00, 1.01)
Latino
Yes
0.9 (0.3, 3.1)
2.5 (1.2, 5.0)
3.2 (1.6, 6.5)
3.0 (1.3, 6.9)
2.0 (0.8, 5.4)
1.5 (0.3, 7.7)
Table 3: Adjusted odds ratios for covariates related to Ishak Fibrosis Stage among HIV/HCV co-infected patients.
Figure 3B presents the mean Fibrosis Progression Rates (FPR), expressed as units/year, in association to the different grades of steatosis. The highest FPR values, 0.21 and 0.24, were of the HIV/ HCV patients with no and mild steatosis, respectively. The lowest FPR value of 0.10 was of the HIV/HCV co-infected patients with severe steatosis. In accordance, these values in the HIV/HCV co-infected patients did not show a statistically significant correlation (p=0.493). In contrast, the values of the HCV mono-infected patients, although of subtle difference, did correlate well although not statistically significant (p=0.088).
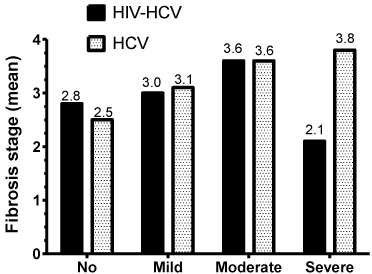
Figure 3A: Mean fibrosis score at different stages of steatosis for HCV monoinfected
and HIV/HCV co-infected patients.
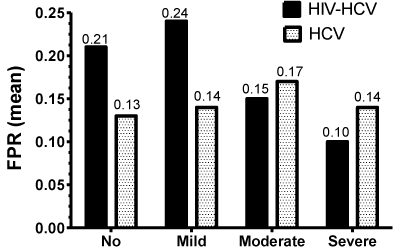
Figure 3B: Mean Fibrosis Progression Rate (FPR) at different stages of
steatosis for HCV mono-infected and HIV-HCV co-infected patients.
Discussion
Hepatic steatosis is caused by the complex interaction of host and viral factors, such as metabolic syndrome, obesity, DM, IR, alcoholism and HCV genotype, and in HIV/HCV co-infected patients, ART may play a role. The finding of 51.2% HS prevalence in our HCV mono-infected patients is quite consistent with reports from previous studies (40-80%) [5,7]. On the other hand, the 39.7% HS prevalence in our HIV/HCV co-infected patients differs from the mean value (50.8%) found in a meta-analysis study [7]; although our results are within the range presented in the study (23-72%). One would expect a higher prevalence of HS in the HIV-infected group since, theoretically, several HIV factors, including the use of ART, have pro-steatogenic effects. Metabolic factors, such as: IR, DM, dyslipidemia and lipodystrophy, are also linked to ART [32,33], and Nucleoside Reverse Transcriptase Inhibitors (NRTI) may cause mitochondrial dysfunction with subsequent direct hepatotoxic effects [34]. Moreover, HIV infection itself may facilitate DM by way of tumor necrosis factor-a stimulation and mitochondria damage [35]. However, in spite of these known pro-steatogenic effects, there have been studies that failed to show an association of HIV-related factors (CD4 count, HIV duration, HIV viral load, use and class of ART) to HS [7,36]. Similar to our findings, another study found only 31% of the HIV-infected patients had HS, and mostly of mild grade (60%) [36]. It is possible that the lower prevalence in the co-infected cohort is due to improved medical management with better control of diabetes, lipidemias and lower alcohol consumption.
Seeking other possibilities for the significantly different prevalence of HS in the two groups, we focused first on the presence of metabolic risk factors. In this study, we have a higher BMI and more alcohol abuse in the HCV mono-infected group, but higher triglycerides and lower HDL levels in the HIV/HCV co-infected group, and the percentage of diabetics, an important risk factor for HS, was similar in both groups. The presence of risk factors is unequal but inconsistent and, therefore, cannot explain the difference of HS prevalence. Regarding the possible role ethnicity plays in the prevalence of HS, studies have indicated that Latinos have a higher risk because their upper prevalence of metabolic syndrome [37]. In this study, we have a significantly higher percentage of Latinos in the HIV/HCV co-infected patients, which is, paradoxically, the group with the lower HS prevalence. Concerning the HCV viral-related factors, one significantly associated with HS is the genotype 3 [38,39]. In this study, the percent of those with genotype 3 was higher in the HIV/HCV co-infected patients (9.8%) versus those infected with HCV alone (3.3%), favoring the group that actually had the lower prevalence of HS. Interestingly, the overall prevalence of genotype 3 in this study was low (5.1%), contrasting with the prevalence in United States (estimated at 4%) [40], and Europe (10% to =50% in Eastern countries) [41].
With regards to the possible impact of HS on liver disease progression, this study revealed that the HS impacted differently in each group. HS was significantly associated with the necroinflammatory activity grade and the fibrosis progression rate in the HCV monoinfected patients, but not in the HIV/HCV co-infected patients. In the multivariate analysis, HS did increase the chances of fibrosis across all levels in the HCV mono-infected group, in addition to AST level and age at liver biopsy. In the HIV/HCV co-infected group, the AST level and age at liver biopsy were also significantly related to all fibrosis stages; however, only moderate HS (stage 2) was significantly related.
In addition to the findings with HS, the fibrosis multivariate analysis revealed a significant but inverse relationship with total cholesterol levels and fibrosis in HCV mono-infected patients, and an inconsistent positive relationship of Latino ethnicity and fibrosis. Latino ethnicity was only significantly related to fibrosis stage 3 to 6 in the HCV mono-infected group and fibrosis stage between 2 and 4 in the HIV/HCV co-infected group.
As discussed, we could not identify a reason to explain why HS is not associated with the progression of fibrosis in the HIV/HCV co-infected patients in this study, as it has been described elsewhere [5,7,42]. We considered the possibility of sampling variability of the liver biopsy [43]. However, we have liver biopsies that are longer than average (35 mm) and from 3 separate directions, for the majority of patients in this study. In addition, liver sampling should be equally relevant to both groups. A possible explanation for this discrepancy could be the medical history for the duration and selection of ART drugs. The duration of use of ART (mean 3.25 years) may be short to observe the progression changes induced by additional steatosis.We may also have more patients that used ART drugs with lower risk of steatosis than others, or patients with lower % of metabolic syndrome manifestations. We know that immune mechanisms play a role in fibrosis progression, and one can hypothesize that the HCV steatogenic effects are so strong that they supersede any HIV related effects. Therefore, as previously discussed, improved management of metabolic syndrome variables in the co-infected cohort may be having a beneficial effect in hepatic steatosis.
In conclusion, this study revealed that HS is relatively common in the HCV mono-infected patients and somewhat less prevalent in the HIV/HCV co-infected patients; but most importantly, its presence impacted differently the progression of liver disease. The HS increased consistently the severity of the necroinflammatory grade, the fibrosis stage, and the fibrosis progression rate in HCV monoinfected patients, but not in HIV/HCV co-infected patients. Finally, given the adverse impact of HS on fibrosis progression, additional studies on the efforts to reduce steatosis by addressing modifiable factors, such as weight, lipid levels, alcohol intake, and the treatment of HCV (especially in those with genotype 3) are needed. These interventions are relevant even when curative drug combinations are available to manage chronic HCV infection. NAFLD associated to HCV or not is expected to become the next challenge to preserve the health of the liver.
References
- Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol. 2005; 42: 2-12.
- Sanyal AJ. American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002; 123: 1705-1725.
- Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006; 43: 682-689.
- Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999; 116: 1413-1419.
- Sterling RK, Contos MJ, Smith PG, Stravitz RT, Luketic VA, Fuchs M, et al. Steatohepatitis: Risk factors and impact on disease severity in human immunodeficiency virus/hepatitis C virus coinfection. Hepatology. 2008; 47: 1118-1127.
- Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005; 288: 462-468.
- Machado MV, Oliveira AG, Cortez-Pinto H. Hepatic steatosis in patients coinfected with human immunodeficiency virus/hepatitis C virus: a meta-analysis of the risk factors. Hepatology. 2010; 52: 71-78.
- Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005; 42: 928-940.
- Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001; 120: 1183-1192.
- Loria P, Lonardo A, Carulli L, Verrone AM, Ricchi M, Lombardini S, et al. Review article: the metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2005; 22: 31-36.
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001; 33: 1358-1364.
- Monto A, Alonzo J, Watson JJ, Grunfeld C, Wright TL. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002; 36: 729-736.
- Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002; 37: 837-842.
- Halfon P, Pénaranda G, Carrat F, Bedossa P, Bourlière M, Ouzan D, et al. Influence of insulin resistance on hepatic fibrosis and steatosis in hepatitis C virus (HCV) mono-infected compared with HIV-HCV co-infected patients. Aliment Pharmacol Ther. 2009; 30: 61-70.
- Merchante N, Rivero A, de Los Santos-Gil I, Merino D, Márquez M, López-Ruz MA, et al. Insulin resistance is associated with liver stiffness in HIV/HCV co-infected patients. Gut. 2009; 58: 1654-1660.
- Castera L, Hezode C, Roudot-Thoraval F, Bastie A, Zafrani ES, Pawlotsky JM, et al. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut. 2003; 52: 288-292.
- Adinolfi LE, Durante-Mangoni E, Zampino R, Ruggiero G. Review article: hepatitis C virus- associated steatosis—pathologic mechanisms and clinical implications. Aliment Pharmacol Ther. 2005; 22: 52–55.
- Urial A, Moorehead L, Agarwal K. Insulin resistance associated with poorer HCV virologic response in HCV/HIV coinfected patients (Abstract 925). 12th Conference on Retroviruses and Opportunistic Infections. Boston, MA. 2005.
- Sulkowski MS, Mehta SH, Torbenson M, Afdhal NH, Mirel L, Moore RD, et al. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005; 19: 585-592.
- Verma S, Goldin RD, Main J. Hepatic steatosis in patients with HIV-Hepatitis C Virus coinfection: is it associated with antiretroviral therapy and more advanced hepatic fibrosis? BMC Res Notes. 2008; 1: 46.
- Rodríguez-Torres M, Bräu N, Rodríguez-Orengo JF. Severity of liver steatosis affects progression of disease in the HCV mono-infected patients but not in the HCV/HIV co-infected patients (Abstract 1633). 60th Annual Meeting of the American Association for the Study of Liver Diseases. Boston. 2009.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995; 22: 696-699.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999; 94: 2467-2474.
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41: 1313-1321.
- Stata Corp. Stata Statistical Software: Release 11. College Station, TX: Stata Corp LP 2009.
- McCullagh P. Regression models for ordinal data. Journal of the Royal Statistical Society. 1980; 42: 109–142.
- Peterson B, Harrell FE. Partial proportional odds models for ordinal response variables. Applied Statistics 1990; 39: 205–217.
- Campbell RT, Li X, Dolecek TA, Barrett RE, Weaver KE, Warnecke RB. Economic, racial and ethnic disparities in breast cancer in the US: towards a more comprehensive model. Health Place. 2009; 15: 855-864.
- Warren JS, Nelson PL, Mondragon SA, Baldwin SA, Burlingame GM. Youth psychotherapy change trajectories and outcomes in usual care: Community mental health versus managed care settings. J of Consulting and Clinical Psychology. 2010; 78, 144–155.
- Ziraba AK, Fotso JC, Ochako R. Overweight and obesity in urban Africa: A problem of the rich or the poor? BMC Public Health. 2009; 9: 465.
- Silva GF, Grotto RM, Verdichio-Moraes CF, Corvino SM, Ferrasi AC, Silveira LV, et al. Human platelet antigen genotype is associated with progression of fibrosis in chronic hepatitis C. J Med Virol. 2012; 84: 56-60.
- Noor MA, Parker RA, O'Mara E, Grasela DM, Currie A, Hodder SL, et al. The effects of HIV protease inhibitors atazanavir and lopinavir/ritonavir on insulin sensitivity in HIV-seronegative healthy adults. AIDS. 2004; 18: 2137-2144.
- Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998; 12: 51-58.
- Walker UA, Bäuerle J, Laguno M, Murillas J, Mauss S, Schmutz G, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology. 2004; 39: 311-317.
- Piroth L. Liver steatosis in HIV-infected patients. AIDS Rev. 2005; 7: 197-209.
- Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009; 50: 464-473.
- Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009; 49: 791-801.
- Rodriguez-Torres M, Govindarajan S, Diago M, Morgan T, Anand B, Barange K, et al. Hepatic steatosis in patients with chronic hepatitis C virus genotype 2 or 3 does not affect viral response in patients treated with peginterferon alpha-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS) for 16 or 24 weeks. Liver Int. 2009; 29: 237-241.
- Rodríguez-Torres M, Govindarajan S, Solá R, Clumeck N, Lissen E, Pessôa M, et al. Hepatic steatosis in HIV/HCV co-infected patients: correlates, efficacy and outcomes of anti-HCV therapy: a paired liver biopsy study. J Hepatol. 2008; 48: 756-764.
- Rustgi VK. The epidemiology of hepatitis C infection in the United States. J Gastroenterol. 2007; 42: 513-521.
- Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008; 48: 148-162.
- Gaslightwala I, Bini EJ. Impact of human immunodeficiency virus infection on the prevalence and severity of steatosis in patients with chronic hepatitis C virus infection. J Hepatol. 2006; 44: 1026-1032.
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005; 128: 1898-1906.
Citation: Rodríguez-Torres M, Bräu N, Rios-Bedoya CF, Hallman D, Maldonado I et al. Hepatic Steatosis and Metabolic Syndrome Variables: Overplay on the Progression of Fibrosis among Hepatitis C Mono-Infected Patients and Human Immunodeficiency Virus Co-Infected Patients. J Hepat Res. 2015;2(1): 1018. ISSN:2381-9057