
Case Report
J Hepat Res. 2015;2(1): 1019.
A Case Report: Small Intestinal GIST
Arun Joyati Tarafder1, Mamun–Al-Mahtab2*, Sisir Ranjan Das3, Madhusudan Shaha4, Izazul Haque5, Rezaul Karim6 and Salimur Rahman1
1Department of Hepatology, Mymensingh Medical College, Bangladesh
2Department of Hepatology, Bangabandhu Sheikh Mujib Medical University, Bangladesh
3Department of Neonatology, Dhaka Medical College, Bangladesh
4Department of Gastroenterology, North East Medical College, Bangladesh
5Department of Hepatology, Comilla Medical College, Bangladesh
6Senior Consultant & Interventional Cardiologist, Cardiac Hospital & Research Institution, Bangladesh
*Corresponding author: Mamun–Al-Mahtab, Department of Hepatology, Mymensingh Medical College, Bangladesh
Received: July 08, 2014; Accepted: March 20, 2015; Published: March 31, 2015
Abstract
Gastrointestinal Stromal Tumor (GIST) is the commonest mesenchymal tumour of the gastrointestinal tract, representing 1-3% of gastrointestinal malignancies. Diagnosis is confirmed on histopathology and immunohistochemistry. Surgery remains the standard initial management for all localized GISTs. We herein report a classical case of a 60 year old male with intestinal GIST.
Keywords: GIST; Gastrointestinal stromal tumor; Malignancy
Introduction
Gastrointestinal Stromal Tumor (GIST) is the most common (80%) mesenchymal tumor of the alimentary cannel, representing 1-3% of gastrointestinal malignancies [1]. GISTs are usually found in the stomach or small intestine but can occur anywhere along the GI tract and rarely have extra-GI involvement [2]. The majority of GISTs present at ages 50-70 years with no clear gender predilection [3]. GISTs are uncommonly seen in patients younger than 40, however, cases in children and young adults have been reported [4]. GISTs are thought to arise from interstitial cells of Cajal (ICC), They are typically defined as tumors whose behavior is driven by mutations in the Kit gene or PDGFRA gene [1] and recognition of KIT protein expression (CD 117) as a reliable diagnostic marker of disease.
Case Report
The patients, 60 years old man, normotensive, non-diabetic, from middle class socio-economic background presented with vague abdominal pain, repeated attack of pallor and something feeling in central abdomen. He had no history of haematemesis and or melaena, cough or breathlessness, chest pain or palpitation and urinary complains. He never experienced jaundice and his bowel habit was normal.
On clinical examination, the patient was ill looking, anaemic, well oriented and co-operative with average built and nutrition. He was not icteric and no bony tenderness, lymphadenopathy and any organomegaly. He had an ill defined mobile rounded mass about 10×8 cm at umbilical region soft to firm in consistency non tender and not fixed with overlying skin. He had no ascites. Others systemic examination revealed no abnormality.
Hematological investigations revealed haemoglobin 8 gm/dl, ESR -80mm/1st hour, platelets 350×109 /L, total count of WBC 6000/mm3. Peripheral blood film showed hypochromic and and normocytic red blood cells, mature WBC and normal platelets count (Figure 1).
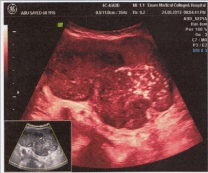
Figure 1: USG shows a heterogeneous mass arising from the distal small
bowel.
He tested negative for HBsAg, anti HCV and stool OBT.
Ultrasonography of whole abdomen revealed heterogenous mass (11.47×8.9×7.1 cm) susceptive of GIST originating from small intestine, no evidence of metastasis.
CT scan of whole abdomen revealed heterogenous mass (11.47× 8.9×7.1 cm) susceptive of GIST originating from small intestine, no evidence of metastasis (Figure 2).
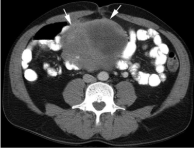
Figure 2: CT scan shows a heterogeneous mass (white arrows) arising from
the distal small bowel.
CT guided FNAC showed cellular material composed of spindly cells arranged in large sheets, clusters and singly. These cells show mild pleomorphism, compatible with spindle cell neoplasm.
Plan X-ray abdomen, endoscopy, colonoscopy revealed normal. He had normal X-ray chest and sinus tachycardia on ECG.
After clinical diagnosis the patients was referred to a surgeon for operative treatment.
Discussion
The majority of GISTs (60%) are seen in the stomach, usually in the fundus. The percentages of GISTs found in other portions of GI tract are reported as 30% in jejunum and ileum, 5% in duodenum, 4% in colorectum, and rarely in the esophagus and appendix [5] are Most GISTs sporadic. Less than 5% occur as part of hereditary familial or idiopathic multitumor syndromes [6]. 70-80% of GISTs were benign [7]. Malignancy is characterized by local invasion and metastases, usually to the liver, omentum and peritoneum. However, cases of metastases to bone, pleura, lungs and retroperitoneum have been seen. In distinction to gastric adenocarcinoma or gastric/small bowel lymphoma, malignant lymphadenopathy is uncommon (<10%) and thus imaging usually shows absence of lymph node enlargement. If metastases are not present, other radiologic features suggesting malignancy include: size (>5 cm), heterogeneous enhancement after contrast administration and ulcerations [8].
Most GISTs remain ‘silent’ until reaching a large size. Symptoms vary according to location and size. Symptomatic GIST patients generally present with nonspecific symptoms including abdominal pain, fatigue, dyspepsia, nausea, anorexia, weight loss, fever and obstruction. Patients may present with chronic GI or overt bleeding due to mucosal ulceration or tumor rupture with life-threatening intraperitoneal hemorrhage. Some patients with large GISTs may have externally palpable masses [4].
The diagnostic evaluation of GISTs is based on imaging techniques, with a special role of endoscopic examination because it is usually accessible when tumors are in the stomach, esophagus and large intestine. In addition, endoscopic ultrasonography (EUS) also plays an important role in the diagnostic work-up of GISTs and is accurate and efficient in the diagnosis of GISTs. In general, externally bilging tumors are more common than intraluminal masses. Punchout ulcer is the classical appearance of a submucosal tumor [2].
A biopsy sample will be investigated under the microscope. Microscopically, GISTs have a broad morphological spectrum. Three main histological subtypes have been best widely accepted and they are spindle cell type (most common, 70%), epithelioid type (20-25%), and mixed spindle cell and epithelioid type. In general, GISTs have a wide variation ranging from hypocellular to highly cellular with higher mitotic rates [9]. Immunohistochemically, the vast majority of GISTs (95%) are strongly and diffusely positive for KIT (CD117), which makes the KIT to be a very specific and sensitive marker in the differentiating GIST from other mesenchyma tumors in the GI tract [10]. Other markers which can be expressed by GISTs include h-caldesmon, SMA, S100, desmin, Vimentin, and cytokeratins 8 and 18. Recently other CD markers for GISTs are reported including CD10 [11], CD133, and CD44 [12].
Although GISTs are the most common mesenchymal tumor of the GI tract, a variety of other tumors should be included in the differential diagnosis. Accurate recognition of GIST is obviously important as the treatment differs according to the tumor type. The main differential diagnoses include smooth muscle tumors, schwannoma, desmoid fibromatosis, inflammatory myofibroblastic tumor, inflammatory fibroid polyp, solitary fibrous tumor, synovial sarcoma, follicular dendritic cell sarcoma, glomus tumor, and melanoma.
The risk of relapse of GISTs is estimated based on mitotic rate, tumor size, tumor site, surgical margins and the status of tumor rupture. Tumor size and mitotic count are considered to be the most useful and best prognostic factors [13].
Conclusion
The rapid expansion of molecular and clinicopathological knowledge of GIST has given this disease a promising future. Molecular testing of GISTs should be performed for treatment selection and assessment of disease progression. The cause of GIST is still unknown; therefore, little has been done preventively. However, with gradual understanding the molecular mechanisms of GIST, the etiology will be elucidated eventually.
References
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006; 130: 1466-1478.
- Zhao X, Yue C. Gastrointestinal stromal tumor. J Gastrointest Oncol. 2012; 3: 189-208.
- Kantarjian HM, Wolff RA, Koller CA. The MD Anderson Manual of Medical Oncology. 2nd edn. McGraw-Hill. 2011.
- Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: a clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005; 29: 1373-1381.
- Quek R, George S. Gastrointestinal stromal tumor: a clinical overview. Hematol Oncol Clin North Am. 2009; 23: 69-78.
- Agaimy A, Hartmann A. Hereditary and non-hereditary syndromic gastointestinal stromal tumours]. Pathologe. 2010; 31: 430-437.
- Burkill GJ, Badran M, Al-Muderis O, Meirion Thomas J, Judson IR, Fisher C, et al. "Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread". Radiology. 2003; 226: 527–532.
- Hersh MR, Choi J, Garrett C, Clark R. Imaging gastrointestinal stromal tumors. Cancer Control. 2005; 12: 111-115.
- Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011; 104: 865-873.
- Hornick JL, Fletcher CD. The role of KIT in the management of patients with gastrointestinal stromal tumors. Hum Pathol. 2007; 38: 679-687.
- Wong NA, Melegh Z. Gastrointestinal stromal tumours can express CD10 and epithelial membrane antigen but not oestrogen receptor or HMB45. Histopathology. 2011; 59: 781-785.
- Chen J, Guo T, Zhang L, Qin LX, Singer S, Maki RG, et al. CD133 and CD44 are universally overexpressed in GIST and do not represent cancer stem cell markers. Genes Chromosomes Cancer. 2012; 51: 186-195.
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002; 33: 459-465.