
Research Article
J Hepat Res. 2015;2(1): 1021.
Hepatitis B Virus Infection: Characteristics of Patients, Frequency and Significance of Viral Markers
Akere A1*, Akande KO2, Oke TO2 and Fakoya TO2
1Department of Medicine, College of Medicine, University of Ibadan/University College Hospital, Nigeria
2Department of Medicine, University College Hospital, Nigeria
*Corresponding author: Akere A, Department of Medicine, College of Medicine, University of Ibadan/ University College Hospital, Ibadan, Nigeria, P.O. Box 28829, Agodi, Ibadan, Nigeria
Received: January 16, 2015; Accepted: April 13, 2015; Published: April 28, 2015
Abstract
Aim: This was to evaluate the characteristics of subjects with chronic HBV infection and describe the pattern of serological markers of the infection.
Methods: A total of 132 subjects was recruited. A standard questionnaire was used to document demographic information of each participant, as well as possible risk factors for HBV infection. The subjects had physical examination and laboratory tests which included Abdominal Ultrasound Scan, Liver Function Tests, HBeAg, antiHBe, antiHCV and HBV DNA assay.
Results: The subjects comprised 89 (67.4%) males and 43 (32.6%) females. The mean age of the patients was 34.0±9.3 years with a range of 13- 57 years. Analysis showed that 50 (37.9%) of them were between 30-39 years. Risk factors for HBV infection identified in the patients were scarification in 26 (19.7%), previous blood transfusion in 8 (6.1%), past surgery in 28 (21.2%), with the most frequent surgery being appendicectomy in 3 (2.3%), followed by dental extraction in 2 (1.5%) patients. Other risk factors were multiple sexual partners in 37 (28%) and indiscriminate injections in 6 (4.5%).
Serology showed 24 (18.2%) patients were HBeAg positive, while 88 (66.7%) were antiHBe positive. Mean ALT was 33.0 ±21.7 iu/ml (range 2-150). Value of ALT ≥ 40 iu/ml was observed in 37 (28%) patients. Mean HBV DNA was 9,284,149.2±36,748,525.5 iu/ml (range of 20-170,000,000 iu/ml). HBV DNA level ≥ 2,000 iu/ml was observed in 54 (40.9%) patients.
Conclusion: In conclusion, markers of HBV infection are prevalent in these asymptomatic subjects. So, there is need to always screen for these markers.
Keywords: Hepatitis B virus; Serum markers; Significance; Characteristics of patients
Introduction
Hepatitis B Virus (HBV) infection is a common disease that constitutes a major public health problem globally. About 5% of the world’s population is infected with this virus [1]. It is a common cause of liver cirrhosis, liver failure, as well as liver cancer which result in about two million deaths annually [2].
There are wide regional variations in the prevalence of HBV infection, with rates greater than 8% seen in Asia, Western Pacific and Africa; regions of intermediate rates (2-7%) are southern and eastern Europe, whereas rates less than 2% are seen in western Europe, Australia and the USA [3,4]. It has been observed that, at least one marker of HBV infection is present in about 70-95% of subjects in sub-Saharan Africa [5].
HBV is a member of the family of hepadnaviridae which infects humans and certain animal species like duck, ground squirrel and woodchuck.6 It is characterized by the presence of partially doublestranded DNA surrounded by an outer lipoprotein and an inner core.6 HBV is compact and contains four Open Reading Frames (ORF) which are S,P,C and X, and these encode four major proteins: surface, polymerase, core and X protein respectively.
Chronic infection with HBV is dynamic and progresses through four major phases which are: immune-tolerant, immune-reactive, inactive phase and HBeAg-negative chronic HBV infection. However, these phases are not necessarily sequential and are important to determine those that require treatment, as well to predict prognosis.
HBV has a complex serology and natural history as a result of multiple serological markers which include HBsAg, antiHBs, antiHBc, HBeAg, antiHBe and HBV DNA quantification.
The aim of this study was to evaluate the characteristics of asymptomatic patients with chronic HBV infection and describe the pattern of serological markers of the infection.
Materials and Methods
This was a descriptive study, which was carried out at the gastroenterology/hepatology outpatient clinic of a tertiary health facility in south-west Nigeria. The subjects were asymptomatic patients who were HBsAg positive. A total of 132 subjects who gave their consent to participate were recruited for the study. A standard questionnaire was used to document demographic information about each participant. Also included in the questionnaire were possible risk factors for HBV infection. All the subjects had physical examination performed on them following which, laboratory tests were ordered and these included Abdominal Ultrasound Scan, Liver Function Tests, HBeAg, antiHBe, antiHCV and HBV DNA assay.
Data were analyzed using SPSS version 17. Values were expressed as means. Association between proportions was compared using chisquare. P value of less than 0.05 was considered significant.
Results
A total of 132 patients, comprising 89 (67.4%) males and 43 (32.6%) females were recruited into the study, giving a M:F ratio of 2.1:1. The mean age of the patients was 34.0±9.3 years with a range of 13-57 years. Analysis of the age groups showed that 50 (37.9%) of them were between 30-39 years, followed by 47 (35.6%) patients who were less than 30 years of age (Figure 1). Student population was 39 (29.5%), followed by professionals who constituted 30 (22.7%). Risk factors for HBV infection identified in the patients were scarification in 26 (19.7%), previous blood transfusion in 8 (6.1%), past surgery in 28 (21.2%), with the most frequent surgery being appendicectomy in 3 (2.3%), followed by dental extraction in 2 (1.5%) patients. Other risk factors were multiple sexual partners in 37 (28%) and indiscriminate injections in 6 (4.5%) (Figure 2). Two (1.5%) patients reported having been vaccinated against HBV infection without prior testing for HBsAg or antiHBc.
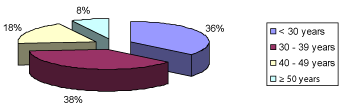
Figure 1: Age group distribution of the patients.
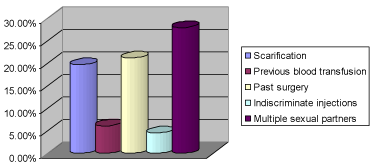
Figure 2: Frequency of HBV risk factors among the patients.
Clinical examination revealed that only 8 (6.1%) patients had hepatomegaly (liver span>12 cm). None of the patients had ascites or splenomegaly.
Abdominal Ultrasound Scan among the subjects
The abdominal ultrasound scan results among the subjects revealed normal study in 98 (74%) subjects, 14 (10.6%) subjects had hepatomegaly but with normal parenchyma, 6 (4.5%) had hepatic steatosis, while another 6 (4.5%) had normal sized liver but with coarse parenchyma. One (0.8%) had normal sized liver with simple hepatic cysts, which was considered incidental.
HBeAg status among the subjects
Serological analysis showed that 24 (18.2%) patients were HBeAg positive, while 88 (66.7%) were antiHBe positive (Figure 3). Among those who were HBeAg positive, 14 (58.3%) of them were less than 30 years of age, whereas 29 (29.3%) of those who were HBeAg negative were less than 30 years of age. There was significant difference between the two groups (p= 0.03). With respect to gender, 16 (19.3%) of them were males, while 7 (17.5%) were females. However, this was not statistically significant (p= 0.81).
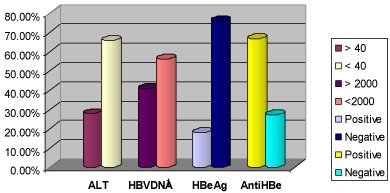
Figure 3: Pattern of ALT and HB viral markers among the patients.
Analysis of the association between HBeAg and the risk factors for HBV infection showed no significant difference between the presence of HBeAg and the majority of the risk factors analysed, except for the history of past surgery where significant difference was observed. (p=0.01) Table 1. The results also showed that, none of the two patients that had vaccination was positive for HBeAg. However, 17 (17.3%) of those with no prior vaccination were positive for HBeAg. But, there was no significant difference between the two groups (p=0.52).
Risk factor
Yes n(%)
No n(%)
p-value
Scarification
5(27.8)
13 (72.2)
0.62
Previous blood transfusion
0 (0)
17(100)
0.21
Past Surgery
0 (0)
17(100)
0.01*
Indiscriminate Injection
1 (5.9)
16(94.1)
0.96
Multiple Sexual Partners
9(47.4)
10(52.6)
0.20
Table 1: Frequency of HBV risk factors among HBeAg positive patients.
Liver Function Tests among the subjects
The mean ALT value was 33.0 ±21.7 iu/ml (range 2-150). Other parameters of the liver function tests are as shown in table 2. Value of ALT ≥ 40 iu/ml was observed in 37 (28%) patients (Figure 3).
Parameter
Mean±SD
Normal range
Total bilirubin (mg/dl)
1.45 ± 2.31
0.2 - 1.0
Conjugated bilirubin(mg/dl)
0.86 ± 1.87
0 - 0.4
ALT (iu/l)
32.96 ± 21.7
0 – 40
AST (iu/l)
35.65 ± 18.44
0 – 37
Alkaline Phosphatase (iu/l)
88.14 ± 32.06
40 – 130 (M)
35 – 105 (F)
Total Protein (g/l)
9.41 ± 11.06
60 – 80
Table 2: Liver Function Tests of the patients.
HBV DNA assay among the subjects
The mean HBV DNA value was 9,284,149.2±36,748,525.5 iu/ml with a range of 20-170,000,000 iu/ml. HBV DNA level ≥ 2,000 iu/ml was observed in 54 (40.9%) patients (Figure 3). Among those with HBV DNA ≥ 2,000 iu/ml, 46 (85.2%) of them were less than 40 years of age and 36 (67.9%) of them were males. It was also observed that the two patients with prior vaccination had serum HBV DNA < 2000 iu/ml, while 45 (43.7%) of those without prior vaccination had serum HBV DNA ≥ 2000 iu/ml. Again, there was no significant difference observed (p=0.22).
Association between HBV DNA and HBeAg status
Among those patients who were HBeAg positive, 21 (87.5%) of them had HBV DNA ≥ 2000 iu/ml, while 30 (30.9%) of those who were HBeAg negative had HBV DNA ≥ 2000 iu/ml and there was significant difference between the two groups (p=0.00).
Association between HBV DNA level and risk factors for HBV infection
As shown in table 3, there was no significant difference between HBV DNA ≥ 2000 iu/ml and exposure to most of the risk factors for HBV infection, except for history of previous blood transfusion where significant difference was observed (p=0.01)
Risk factor
Yes n(%)
No n(%)
p-value
Scarification
9 (19.6)
37 (80.4)
0.42
Previous blood transfusion
0 (0)
45 (100)
0.01*
Past Surgery
8 (17.8)
37(82.2)
0.11
Indiscriminate Injections
3 (6.7)
42 (93.3)
0.75
Multiple Sexual Partners
14 (30.4)
32 (69.6)
0.40
Table 3: Frequency of HBV risk factors among patients with HBV DNA ≥ 2000 iu/ml.
Association between HBeAg status and ALT value
Fifteen (65.2%) of the patients with positive HBeAg had ALT < 40 iu/ml, while 8 (34.8%) of them had ALT < 40 iu/ml. Significant difference was observed between the two groups (p= 0.00).
Association between HBV DNA level and ALT value
The values of HBV DNA and ALT were also compared and it was found that, 18 (35.3%) of those with HBV DNA ≥ 2000 iu/ml had ALT < 40 iu/ml, while 33 (64.7%) of them had ALT < 40 iu/ml. No significant difference between the two groups (p=0.28).
Association between HBeAg, HBV DNA level and ALT value
It was also found that 14 (70%) of those with positive HBeAg and HBV DNA ≥ 2000 iu/ml had ALT < 40 iu/ml, while 6 (30%) had ALT < 40 iu/ml. Significant difference was observed between the two groups (p=0.00).
Discussion
This study revealed that males are more infected with HBV infection than females. This finding is consistent with earlier reports where higher infection rates were found among males [7-12]. One possible explanation for this finding might be because, males are more exposed to the possible risk factors for HBV infection than females.
This study also revealed that 73.5% of the subjects were less than 40 years of age. This again is consistent with reports from other studies where, young people were found to be predominantly infected [7,13]. Although, it is believed that neonates and young children are mostly infected in the developing countries like ours due to perinatal or early childhood transmission [6]. It is however possible that most of these subjects got infected in childhood and had carried the infection to early adulthood without developing any sequelae, since the time of actual infection cannot not be adequately determined in most cases. Another explanation may be that, it is this age group that frequently presents to the hospital either for medical certification or for blood donation, during which they are tested for HBV infection. This is evident from the fact that, all the subjects in this study were asymptomatic, but was found to have HBV infection incidentally either during prescreening for blood donation or during medical fitness test. Another explanation is that sexual activity and injection drug use, which are some of the risk factors for HBV infection, are highest among this age group. This is supported by the fact that, among the risk factors evaluated in this study, history of multiple sexual partners was the commonest among the subjects. It has been observed that, sexual transmission may be an important mode of transmission all over the world, since many patients with chronic HBV infection are asymptomatic and unaware of their infection [6].
The HBeAg prevalence of 18.2% obtained in this study is higher than the 2.3% reported by Otegbayo et al. [14] in a study among blood donors at the same site. However, only 37(21.3%) of their subjects were HBsAg positive, whereas all the subjects in this present study were HBsAg positive. This difference in the number of subjects with HBV infection might explain the low HBeAg recorded in that study.
But, in a study by Onyekwere et al. [15] in Lagos which is located in the same geopolitical zone where our study was carried out, a higher prevalence (57%) of HBeAg was reported in a control group, whereas none of the diabetes patients, who were the main subjects of the study, was positive for HBeAg. The reason for this high prevalence in the control group is not known, but one would have expected some of the diabetes patients to be HBeAg positive considering the fact that diabetes is associated with immunosuppression.
In another study conducted by Odimayo et al. [16] a lower prevalence of 3.1% for HBeAg was recorded. This may reflect the overall low prevalence of HBV infection (96/467) in the population studied, whereas all the 132 (100%) subjects in our study had HBV infection.
Lesi et al. [17] reported HBeAg prevalence of 27.3% which is also higher than our figure. However, this was found in HIV/AIDS patients who had HBV co-infection. The presence of immunosuppression in this category of patients might explain the higher prevalence of HBeAg positivity.
In Ghana, another country in the West African sub region, HBeAg prevalence of 13.3% was reported by Rufai et al. [8]. The sample size in this particular study was comparable to ours and all the subjects were HBsAg positive
In our study, it was observed that HBeAg was significantly more prevalent in younger subjects, whereas anti-HBe was more prevalent in older subjects. This is consistent with the findings of Mendy et al. [18] in the Gambia where clearance of HBeAg was found to occur at steady rates over the years. It has been observed that early in the course of HBV infection, younger patients are HBeAg positive, whereas older patients are anti-HBe positive which appears after HBeAg seroconversion [19].
In this study, significant association was observed between positive HBeAg and elevated Alanine Transaminase (ALT) above the upper limit of normal. This may imply that HBeAg, which is both a marker of viral replication and infectivity could also be a marker of ongoing hepatic necroinflammation as reflected by raised ALT. This observation was also reported by Otegbayo et al. [14] where elevated ALT value was seen in all the HBeAg positive subjects.
This study also revealed that positive HBeAg significantly correlates with elevated HBV DNA value. It is known that HBV DNA, just like HBeAg also connotes active viral replication and infectivity, and so some level of correlation is expected between these two parameters. But, in clinical practice this is not always observed. Sometimes, high level of HBV DNA may be observed in HBeAg negative subjects and vice versa. This is one of the situations when liver biopsy may be indicated to resolve the disparity. However, one should bear in mind the existence of HBeAg negative chronic hepatitis B, which occurs as a result of mutation in the pre-core and basal core promoter regions of the virus. These mutant forms are unable to express HBeAg, even when HBV DNA level is very high. The existence of these mutant forms of HBV in our environment is difficult to ascertain because, most of those subjects who were HBeAg negative had anti-HBe. The presence of anti-HBe probably indicates that HBeAg was present at some point in the course of the infection.
The range of HBV DNA observed in this study is wider and higher than that reported by Okwuraiwe et al. [19] However, in both studies higher values were seen in males compared to females. In that particular study, the highest value of HBV DNA was reported in those 0-9 years of age, whereas in this present study, the highest value was seen in those less than 40 yrs of age with the youngest being 13 years of age. The finding of higher HBV DNA in younger subjects is consistent with the finding of Mendy et al. [19] where HBV DNA levels were observed to decline with age.
In contrast to what was obtained with HBeAg positivity and active liver disease, HBV DNA > 2000 iu/ml was not associated with active liver disease in the majority (64.7%) of subjects. Although, there is no immediate explanation for this, but a combination of positive HBeAg and HBV DNA > 2000 iu/ml resulted in statistically significant active liver disease (ALT > 40 iu/l) in higher number of subjects (70%). It may therefore imply that, positive HBeAg alone or in combination with HBV DNA > 2000 iu/ml is associated with active liver disease, as indicated by elevated ALT > 40 iu/l.
Another observation in this study is that, those two subjects who had prior HBV vaccination were HBeAg negative and had HBV DNA < 2000 iu/ml. Although, vaccination for HBsAg positive individuals is not recommended, these individuals received their HBV vaccination without knowing their HBsAg status. Probably, the vaccination might have had a positive effect on these individuals as reflected in their HBeAg status and HBV DNA value. However, this practice should be discouraged. Only those individuals who are HBsAg and antiHBc negative should be recommended for HBV vaccination, while Hepatitis B Immunoglobulin (HBIG) should be used for postexposure prophylaxis.
Conclusion
In conclusion, markers of HBV infection are prevalent in asymptomatic subjects, especially in younger subjects. This further supports the need to always evaluate asymptomatic subjects with chronic HBV infection for viral markers. It also underscores the importance of HBV DNA assay even when HBeAg is negative.
References
- World Health Organization. Hepatitis B fact sheet No 204. 2009.
- Clement CJ, Kane M, Hu DJ, Kim-Farley R. Hepatitis B vaccine joins fight against pandemic disease. World Health Forum. 1990; 11: 165-168.
- McMahon BJ, Rhoades ER, Heyward WL, Tower E, Ritter D, Lanier AP, et al. A comprehensive programme to reduce the incidence of hepatitis B virus infection and its sequelae in Alaskan natives. Lancet. 1987; 2: 1134-1136.
- Margolis HS, Alter MJ, Hadler SC. Hepatitis B: Evolving epidemiology and implications for control. Sem in Liver Dis. 1991; 11: 84-92.
- Kire CF. The epidemiology and control of hepatitis B in sub-Saharan Africa. Prog Med Virology. 1993; 40: 143-56.
- Berenguer M, Wright TL. Viral Hepatitis. Sleisenger MH, Fordtran JS, editors. In: Gastrointestinal and Liver Disease, Pathophysiology/Diagnosis/ Management. 7th edn. Volume 2. Philadelphia: Saunders. 2002; 1285-1289.
- Adoga MP, Gyar SD, Pechulano S, Bashayi OD, Emiasegen SE, Zungwe T, et al. Hepatitis B virus infections in apparently healthy urban Nigerians: data from pre-vaccination tests. J Infect Dev Ctries. 2010; 4: 397-400.
- Rufai T, Mutocheluh M, Kwarteng K, Dogbe E. The prevalence of hepatitis B virus e antigen among Ghanian blood donors. Pan Afr Med J. 2014; 17: 53.
- Otegbayo JA, Taiwo BO, Akingbola TS, Odaibo GN, Adedapo KS, Penugonda S, et al. Prevalence of hepatitis B and C seropositivity in a Nigerian cohort of HIV-infected patients. Ann Hepatol. 2008; 7: 152-156.
- Jombo GT, Egah DZ, Banwat EB. Hepatitis B virus infection in a rural settlement of northern Nigeria. Niger J Med. 2005; 14: 425-428.
- Okpalugo CE, Oguntibeju OO. Prevalence of human immunodeficiency virus and hepatitis B virus in preoperative patients: Potential risk of transmission to health professionals. Pakistan Journal of Biological Sciences. 2008; 11: 298-301.
- Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, et al. Hepatitis B infection is highly endemic in Uganda: findings from a National serosurvey. Afr Health Sci. 2009; 9: 98-108.
- Ijoma UN, Nwokediuko SC, Onyenekwe B, Ijoma CK. Low prevalence of Hepatitis B “E” Antigen in Asymptomatic Adult Subjects with Hepatitis B Virus Infection in Enugu, South East Nigeria. The Internet Journal of Gastroenterology. 2010; 10: Number 1.
- Otegbayo JA, Fasola FA, Abja A. Prevalence of hepatitis B surface and e antigens, risk factors for viral acquisition and serum transaminase among blood donors in Ibadan, Nigeria. Trop Gastroenterol. 2003; 24: 196-197.
- Onyekwere CA, Anomneze EE, Wali SS. Prevalence of serological markers of chronic hepatitis B virus infection in diabetics in the Lagos University Teaching Hospital, Lagos. Niger Postgrad Med J. 2002; 9: 129-133.
- Odimayo MS, Nwadioha SI, Nwokedi EP. Prevalence of HBeAg among hepatitis B seropositive individuals in Makurdi, Nigeria. American Journal of Biological, Chemical and Pharmaceutical Sciences. 2013; 1: 90-95.
- Lesi OA, Kehinde MO, Oguh DN, Amira CO. Hepatitis B and C virus infection in Nigerian Patients with HIV/AIDS. Niger Postgrad Med J. 2007; 14: 129-133.
- Mendy ME, McConkey SJ, Sande Van der MAB, Crozier S, Kaye S, Jeffries D, et al. Changes in viral load and HBsAg and HBeAg status with age in HBV chronic carriers in The Gambia. Virol J. 2008; 5: 49.
- Okwuraiwe AP, Salu OB, Onwuamah CK, Amoo OS, Odunukwe NN, Audu RA. Experience with hepatitis B viral load testing in Nigeria. African Journal of Clinical and Experimental Microbiology. 2011; 12: 101-105.