
Case Report
J Hepat Res. 2015;2(2): 1023.
Undiagnosed Vertically Acquired HCV Infection Leading to Hepatocellular Carcinoma and Liver Transplant in an Adolescent Female: A Case Report
Andrea Barreto¹, Amanda Fifi¹, Claudia Rojas² and Aymin Delgado-Borrego3,4*
1Division of Pediatric Gastroenterology, Hepatology and Nutrition.University of Miami, Miller School of Medicine, USA
2Division of Pediatric Pathology, University of Miami, Miller School of Medicine, USA
3KIDZ Pediatric Multispecialty Center, Division of Gastroenterology, Hepatology and Nutrition, USA
4Department of Pediatric Gastroenterology, Boston Children’s Hospital, USA
*Corresponding author: Aymin Delgado-Borrego, Pediatric Gastroenterologist and Hepatologist, Division of Pediatric Gastroenterology, Hepatology and Nutrition, KIDZ Pediatric Multispecialty Center, 2600 Immokalee Road Naples, FL 34110, USA
Received: April 23, 2015; Accepted: June 19, 2015; Published: June 24, 2015
Abstract
Hepatitis C virus (HCV) infection remains a global health concern and is the most common blood-borne infection in the United States (U.S.). According to results obtained from the Third National Health and Nutrition Examination Survey, 0.6% of children and adolescents 6 to 19 years of age were found to be infected with HCV in the U.S.However, it is important to note that the majority of children infected with HCV have not been diagnosed. Hepatocellular Carcinoma (HCC) is uncommon in chronically infected children, with few reported cases in the literature. This case describes the development of HCC in an otherwise healthy individual who acquired HCV through vertical transmission and highlights the importance of vigilance of this infection to prevent delay in diagnosis and institution of appropriate management.
Keywords: Hepatitis C; Hepatocellular carcinoma
Case Presentation
The patient, H.S., is a 17-year-old child of an HCV infected mother. She has a history of psoriasis treated topically and nephrolithiasis treated with lithotripsy. Her mother died from complications of HCV infection when the patient was 10 years old and her father has Chronic Hepatitis C (CHC) infection with chronic liver failure.
H.S. presented in May 2011 to the emergency room (ER) complaining of generalized weakness. On physical examination, she had minimal jaundice and ascites, and laboratory testing revealed elevated liver enzymes and a prolonged Prothrombin Time/ International Normalized Ratio (PT/INR). She was admitted to the inpatient floor for further evaluation and tested positive for HCV antibodies, infection was later confirmed by Polymerase Chain Reaction (PCR) and genotyping (initial viral load was 9 million IU/ml, genotype 1a) (Figure 1). She underwent a liver biopsy that revealed cirrhosis. Initially, there was no immediate treatment plan and no communication between clinicians who established the diagnosis and those with the expertise to treat her. H.S. contacted a social worker at her high school in search of a physician to treat her infection. The social worker called the Miami Dade Public Health Department who ultimately referred her to a pediatric hepatologist at Holtz Children’s Hospital. H.S was seen in clinic in June 2011 and was found to be stable. Further laboratory testing was done at the time including assessment of her immunization status to hepatitis A and B. Abdominal ultrasound (US) with Doppler was consistent with cirrhosis and ascites with no evidence of focal masses. She was started on furosemide and spironolactone.
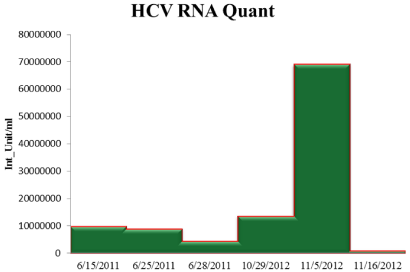
Figure 1: Comparison of HCV viral load as H.S. disease progressed.
Abbreviations: HCV= Hepatitis C Virus; RNA= Ribonucleic Acid
Two weeks later, on June 25th 2011, H.S. presented to the ER at Holtz Children’s Hospital complaining of abdominal distension and paresthesia over the right thigh. She had an oral temperature of 37.8 C., a non-tender, globally distended abdomen with positive shifting dullness, and decreased sensation to light touch and pinprick over her anterolateral right thigh. Her physcial examination was otherwise normal. Her laboratory results are shown in Table 1. An US was performed with the following findings: 1. Abdominal and pelvic ascites, 2. Cirrhotic appearing liver with splenomegaly, ascites and dilated main portal vein suggesting portal hypertension, 3. All imaged hepatic vessels are patient with the appropriate directional flow, 4. Diffusely thickened gallbladder walls, which may be related to the assets and to the partial contraction. She was admitted for fluid management and further work up. During this hospitalization her coagulation studies continued to be abnormal, her enzymes remained elevated, her albumin and vitamin D were low and she was found to have high Thyroid-Stimulating Hormone (TSH) levels (Figure 2). She was human immunodeficiency virus (HIV) negative. The patient was started on albumin with intravenous furosemide and levothyroxine. US of her thyroid was normal. She received transfusions with fresh frozen plasma and cryoprecipitate in preparation for endoscopy, which revealed a single grade 1 vary x that was not bleeding. She had no history of gastrointestinal bleed. No intervention was done at this time. H.S. was started on nadolol prophylactically at 20mg daily, but complained of dizziness so the dose was decreased to 10mg daily. Interferon therapy was contraindicated given her decompensated cirrhosis. Upon presentation, Alpha-Feto Protein (AFP) was mildly elevated (194 ng/ml) and this elevation persisted, but a triple phase Computed Tomography (CT) scan done during admission did not reveal any masses within the liver. In the meantime, pre-transplant work up was initiated and on the 10th day H.S. was discharged on furosemide, spironolactone, nadolol, levothyroxine and sucralfate with plan for follow up with adult gastroenterology in preparation for liver transplantation.
15-Jun
24-Jun
25-Jun
26-Jun
27-Jun
28-Jun
29-Jun
2-Jul
3-Jul
4-Jul
11-Aug
Hb (g/dl)
12.4
12.7
11.4
10.9
10.5
10 g/dl -
HTC
39
38.9
35
33
31.5
WBC
7.8
8.8
6.2
5
6.7
PLT (x3/UL)
84
109
66
67
86
86 x3/UL -
AST (IU/L)
116
87
83
ALT (IU/L)
116
88
74
Glucose (mg/dl)
60
71
94
106
69
83
86
Total bilirubin (mg/dl)
3.2
2.3
2
2 mg/dl +
Direct bilirubin (mg/dl)
1.8
1.2
Total Protein
6.9
7.2
6.7
Albumin (mg/dl)
2.6
2.5
1.9
2.3
3.2
2.6
3.1 mg/dl -
BUN (mg/dl)
5
4
4
3
4
6
Creatinine
0.7
0.77
0.66
0.66
0.7
0.69
PT/INR
19.5/1.7
22.6/1.97
19.5/1.71
16.9/1.47
16.8/1.46
PTT
48
46.1
47.1
42.6
42.4
Fibrinogen
70
125
123
Calcium (mg/dl)
8.6
8.1
8.1
8.1
8.8
8.8
Vitamin D 25 (OH) (ng/mL)
18
18 IU/L -
Vitamin D 1,25 (OH) (ng/mL)
16
Amonia
31
ALP (IU/L)
201
165
108
GGTP (IU/L)
95
85
AFP (ng/ml)
194
184.7
184 ng/ml +
Total Colesterol
105
LDL-C
47
HDL-C
24
Triglycerides
172
ANA
Negative
Positive (40)
Anti-HAV
Negative
Negative
Anti-HBs
Positive
Positive
HCV RNA PCR (IU/mL)
9,868,776
8,937,793
Abbreviations: Hb= Hemoglobin; HTC= Hematocrit; WBC= White blood cell; PTL= Platelets; AST= Aspartate transaminase; ALT= Alaline transaminase; BUN= Blood urea nitrogen; PT/ INR= Prothrombin time/ International normalized ratio; PTT= Patial thromboplastin time; ALP= Alkaline phosphatase; GGTP= Gamma-Glutamyl transpeptidase; AFP= Alfa-fetoprotein;
LDL-C= Low density lipoprotein; HDL-C= High density lipoprotein; ANA= Antinuclear antibody; Anti-HAV= Hepatitis A antibodies; Anti-HBs= Hepatitis B surface antibody.
HCV RNA PCR= Hepatitis C virus Ribonucleic acid Polymerase chain reaction.
Table 1: Laboratory summary.
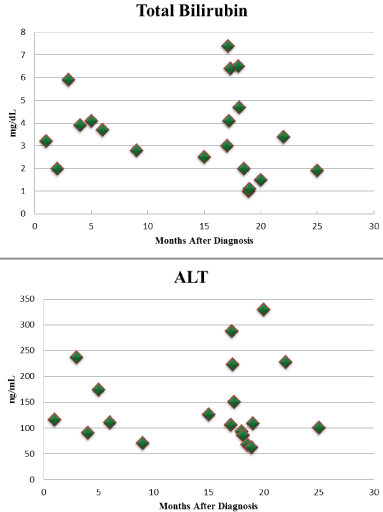
Figure 2: Comparison of Total Bilirubin and ALT levels since H.S.initial
diagnosis.
Abbreviations: ALT: Alanine Transaminase
In august 2011, during a follow up visit, H.S. reported feeling dizzy and fatigued. Physical examination revealed hypotension (blood pressure, 82/63mmHg) and she had conjunctival pallor and mild thyromegaly. Her laboratory results are presented in Table 1. Her ascites was well compensated; therefore, decision was made to decrease the doses of furosemide and spironolactone. Nadolol was continued. She was hepatitis B immune but was not immune against hepatitis also was given hepatitis A vaccine. A multivitamin and vitamin D supplementation were prescribed. Antiviral treatment for hepatitis C was deferred. In addition, due to persistently elevated AFP, a plan was made to repeat imaging every 6 months (Figure 3).
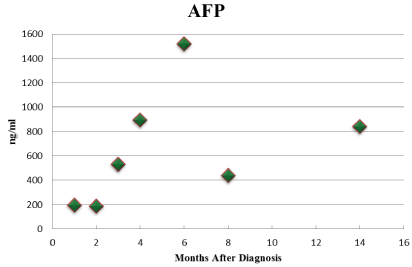
Figure 3: Comparison of AFP levels since initial diagnosis.
By July 2012, 13 months following initial presentation, her CT scan revealed a 2.3 x 2.3 cm mass within the second segment of her liver, suspicious for HCC (Figures 4 and 5).
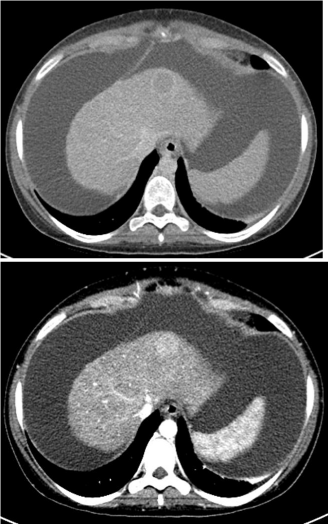
Figure 4 and 5: CT Triple phase of Liver (0.3 mm cuts).
Presence of a 2.3 x 2.3 cm mass within the second segment of Liver.
The patient underwent liver transplantation in October 2012. She had an orthotopic liver transplantation with biliary reconstruction and choledochojejunostomy, Roux-en-Y anastomosis and umbilical hernia repair. Pathologic evaluation confirmed the diagnosis of HCC and her staging was AJJ PT1 N0 M n/a. (Figures 6, 7 and 8). She tolerated the procedure well and was transferred in stable condition to the intensive care unit (Table 2).
Day 0
Tachycardia and apneic episodes. Low urinary output. Lasix was started.
Day 1
Pulmonary edema ocurred and supplementary oxygen was needed.
Day 2
Doppler sonogram performed and showed good vessel patency.
Days 3-4
No events.
Day 5
Diet advanced to regular and the patient had a bowel movement.
Day 6-8
A chest x-ray was done and showed interstitial infiltrates, later on, they improved.
Day 9
Central line was removed, the patient was stable and discharged with the following medical management: aspirin, colace, nexium, lasix, methylprednisolone, nifedipine, nystatin swishand swallow, bactrim,actigall and valcyte.
Table 2: Post-operative evolution.
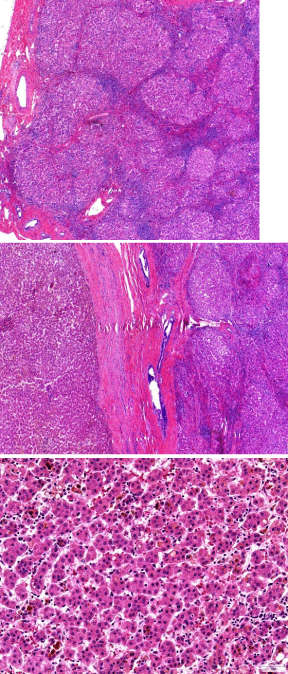
Figure 6,7,8: Pathologic evaluation of H.S. liver.
A diffuse process of the normal lobules has been replaced by architectural
abnormal nodules separated by fibrous tissue showed in Figure 6
corresponding to cirrhosis. The tumor was encapsulated and arising from a
cirrhotic liver (Figure 7). The cells of the hepatocellular carcinoma resemble
normal liver cells, with a trabecular pattern, distinct cell membranes with
moderate amount of eosinophilic, granular cytoplasm, slight high nuclear/
cytoplasm ratio with nuclear irregularity (Figure 8).
During her post-transplant care, her immunosuppressive regimen was limited per protocol to prevent HCV recurrence. Nevertheless, a liver biopsy in February 2014, less than 2 years post-transplant, revealed recurrent hepatitis C, mild to moderate cellular rejection, and moderate fibrosis. She was started on simeprevir/sofosbuvir therapy from March 2014 to June 2014. Her most recent viral load was not detectable in August 2014; 11 weeks post completion of therapy. H.S.’s liver enzymes remain marginally elevated with good liver function and she is currently on tacrolimus and metoprolol. She was also diagnosed with major depression and started on citalopram.
Discussion
Early identification and treatment of hepatitis C infection is beneficial because children who develop chronic hepatitis are at risk of cirrhosis and malignancy. We describe the case of an otherwise healthy HCV infected adolescent who developed decompensated cirrhosis and HCC ultimately requiring a liver transplantation.
H.S.’s case illustrates failure of the system to adequately screen, diagnose and offer treatment to a child born to HCV infected parents with an HCV infected mother who had succumbed to her disease. It was not until H.S. developed symptoms of end stage liver disease that she presented to the ER and was eventually diagnosed. This confirms past reports that HCV infection in pediatrics is largely under diagnosed [1]. Early diagnosis and appropriate follow up would have made H.S. a candidate for medical treatment with the combination approved by the Food and Drug Administration (FDA) for children including pegylated-interferon and ribavirin, which achieves approximately 50%, sustained virological response [2]. Current approved therapies for adults, expected to be available for children in the near future, achieve significantly higher SVR rates, above 90% in many cases, and are associated with minimal side effects. Failure to diagnose and link H.S. To appropriate care resulted in a life threatening condition, with lifelong consequences and led to high medical costs, as the patient ultimately required liver transplantation and lifelong medical management. Although the economic cost of H.S.’s management can be quantified, it is not possible to determine the human impact of this preventable situation on the life of a bright adolescent like H.S. andonsociety.
HCC is extremely uncommon in children with HCV infection and children rarely require liver transplantation for HCV infection [3]. Two cases have been reported as developing HCC in adolescence, one attributable to HCV infection acquired after a stem cell transplant for recurrent acute myelogenous leukemia and the second as a result of perinatal transmission [4]. Two further cases presented as young adults with HCC, both of them due to HCV acquired after blood transfusions [5]. Additionally, one case was reported as a 13-yearold patient with HCV and multifocal HCC who underwent liver transplantation and one month after transplant he experienced significant hepatitis C recurrence, treated with peg–interferon and ribavirin, followed by addition of boceprevir, which led to viral clearance [6]. However, the authors believe that such cases may be more common than previously recognized. Cases such as that of H.S. are likely missed as patients may present with cirrhosis in early adulthood and may not be included in pediatric reports. Failure of medical practitioners to consider the long term consequences of inadequate screening and linkage to care of children at risk for hepatitis C infection has, in turn, clouded our ability to recognize cases that may present in adulthood but are the consequence of vertical HCV transmission. Furthermore, the reported outcome of young patients with HCV-induced HCC has been poor. Among the cited case reports, one died months after transplant, another died of bleeding during removal of a nonfunctional liver transplant [4], and two others died while waiting on a liver transplant [5].
The worldwide financial burden of HCV infection is high, with projected direct medical costs of $10.7 billion from 2010 to 2019 [7,8], and $119-336 million in children over the next decade [9]. Adequate surveillance of children at risk for HCV infectionis necessary to reduce morbidity and mortality and thus reduce the medical costs and social burden of HCV infection and its complications [10].
Conclusions/ Recommendations
HCC secondary to vertical HCV infection can occur in the first 20 years of life in an otherwise healthy individual. From a public health standpoint, this case demonstrates failure of the health care system to diagnose and treat a patient whose parents were chronically infected with HCV and whose mother died of the disease. The patient’s outcomes were most likely preventable given the high rates of sustained virologic response with current antiviral therapy.
References
- Delgado-Borrego A, Smith L, Jonas M, Hall CA, Negre B, Jordan SH, et al. Expected and actual case ascertainment and treatment rates for children infected with hepatitis C in Florida and the United States: epidemiologic evidence from statewide and nationwide surveys. J Pediatr. 2012; 161: 915-921.
- Fish EN, Harrison SA, Hassanein T. The role of consensus interferon in the current treatment of chronic hepatitis C viral infection. Gastroenterol Hepatol (N Y). 2008; 4: 1-12.
- Mack CL, Gonzalez-Peralta RP, Gupta N, Leung D, Narkewicz MR, Roberts EA, et al. NASPGHAN practice guidelines: Diagnosis and management of hepatitis C infection in infants, children, and adolescents. J Pediatr Gastroenterol Nutr. 2012; 54: 838-855.
- González-Peralta RP, Langham MR Jr, Andres JM, Mohan P, Colombani PM, Alford MK, et al. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. J Pediatr Gastroenterol Nutr. 2009; 48: 630-635.
- Strickland DK, Jenkins JJ, Hudson MM. Hepatitis C infection and hepatocellular carcinoma after treatment of childhood cancer. J Pediatr Hematol Oncol. 2001; 23: 527-529.
- Malik S, Dekio F, Wen JW. Liver transplantation in a child with multifocal hepatocellular carcinoma hepatitis C and management of post-transplant viral recurrence using boceprevir. Pediatr Transplant. 2014; 18: E64-68.
- Karnsakul W, Alford MK, Schwarz KB. Managing pediatric hepatitis C: current and emerging treatment options. Ther Clin Risk Manag. 2009; 5: 651-660.
- Lavanchy D. The global burden of hepatitis C. Liver Int. 2009; 29 Suppl 1: 74-81.
- Jhaveri R, Grant W, Kauf TL, McHutchison J. The burden of hepatitis C virus infection in children: estimated direct medical costs over a 10-year period. J Pediatr. 2006; 148: 353-358.
- El-Shabrawi MH, Kamal NM. Burden of pediatric hepatitis C. World J Gastroenterol. 2013; 19: 7880-7888.