
Research Article
J Hepat Res. 2015; 2(3): 1031.
Examination of Centrilobular Necrosis in Autoimmune Hepatitis using Bayesian Networks
Toshiharu Matsumoto¹*, Kanako Ogura1, Asumi Sakaguchi¹ and Toshio Morizane²
¹Department of Diagnostic Pathology, Juntendo University Nerima Hospital, Japan
²Japan Council for Quality Health Case, Japan
*Corresponding author: Toshiharu Matsumoto, Department of Diagnostic Pathology, Juntendo University Nerima Hospital, Nerima-ku, Tokyo, Japan
Received: October 17, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Aim: Autoimmune Hepatitis (AIH) is a chronic portal-based hepatitis. Centrilobular Necrosis (CN) occasionally occurs in AIH, but the participation of CN in inflammatory processes of AIH is not clearly understood. So, in the current study, the significance of CN in AIH was examined with Bayesian Networks (BNs), comprising a newly developed statistical model.
Methods and Results: Sixty AIH patients undergoing liver biopsy examination were evaluated. The significance of CN was examined with BNs using a scoring system for various histological factors, the stage, sex, and age. In all cases, interface hepatitis was present. CN occurred in 13 patients, and 4 of them had pan-acinar necrosis. BNs indicated that interface hepatitis, which became a base factor, was related to portal inflammation, plasmacytic infiltrate, rosette formation of hepatocytes, and focal necrosis, but it was not related to CN.
Conclusion: In AIH, interface hepatitis-related inflammation occurs, and CN occasionally overlaps. When CN progresses to pan-acinar necrosis, a clinically serious condition occurs in the patients. CN is caused by a mechanism that differs from interface hepatitis-related inflammation. The recognition of these is useful for the diagnosis and treatment of AIH.
Keywords: Autoimmune hepatitis; Centrilobular necrosis; Bayesian networks
Abbreviations
AIH: Autoimmune Hepatitis; CN: Centrilobular Necrosis; BNs; Bayesian Networks; DAGs; Directed Acyclic Graphs
Introduction
Autoimmune Hepatitis (AIH) is a chronic portal-based hepatitis with prominent plasma cells [1,2]. Centrilobular Necrosis (CN) occasionally occurs in AIH [3-7]. Recently, CN in AIH was thought to be a histological feature of acute clinical presentation [7]. However, the participation of CN in inflammatory processes of AIH is not clearly understood. Bayesian Networks (BNs) comprise a newly developed probabilistic graphical model (a type of statistical model) that represents a set of random variables and their conditional dependences via Directed Acyclic Graphs (DAGs) [8,9]. Therefore, in the current study, the participation of CN in inflammatory processes of AIH was examined with BNs.
Materials and Methods
Sixty patients who underwent liver biopsy examination in Juntendo University Nerima Hospital and Juntendo Hospital were evaluated. They were diagnosed with AIH according to the international AIH group report [1], and all examined patients had the classic type of AIH. The examined patients were 60 Japanese (5 males and 55 females) with ages ranging from 15 to 77 years (mean: 55 years).
The liver tissues were fixed in 10% formalin and routinely processed for light microscopy. Tissue sections were stained with Hematoxylin-Eosin (HE), reticulum, and Azan stains, and histological findings were evaluated.
The significance of CN was examined with BNs with the bnlearn R package [8,9]. We used hill-climbing method to select the mostly fit network [8]. BNs examination was performed with a scoring system for various histological factors relating to AIH, the stage of AIH, sex, and age. The scores of interface hepatitis (0-4), focal necrosis (0-4), portal inflammation (0-4), and stage (0-6) were according to the scoring systems of chronic hepatitis [10]. The scores of CN were assigned by the modification of confluent necrosis of the scoring systems of chronic hepatitis [10]: 0, absent; 1, CN in a few to some areas; 2, CN in most areas; 3, CN + occasional portal-central bridging necrosis; 4, CN + multiple portal-central necrosis; 5, pan-acinar necrosis. Scores for rosette formation of hepatocytes were defined as follows: 0, absent; 1, mild; 2, severe. Scores for plasmacytic infiltrate were defined as follows: 0, absent; 1, mild; 2, moderate; 3, severe.
The current study was approved by the Research Ethics Committee of Juntendo University Nerima Hospital and Juntendo University School of Medicine.
Results
The incidences of CN, interface hepatitis, portal inflammation, and plasmacytic infiltrate, rosette formation of hepatocytes, focal necrosis, and stage in the examined patients are shown in Table 1. In all patients, interface hepatitis was present (Figure 1). CN occurred in 13 patients (13/60, 21.6%) and 4 of the 13 (30.7%) had panacinar necrosis (Figures 2 and 3). DAGs from BNs indicated that interface hepatitis, which became a base factor, was related to portal inflammation, plasmacytic infiltrate, rosette formation of hepatocytes, and focal necrosis, but it was not related to CN (Figure 4).
Factors
Grade (no. of cases)
CN
0 (47), 1 (2), 2 (3), 3 (2), 4 (2), 5 (4)
Interface hepatitis
0 (0), 1 (12), 2 (20), 3 (9), 4 (19)
Portal inflammation
0 (0), 1 (8), 2 (18), 3 (13), 4 (21)
Plasmacytic infiltrate
0 (0), 1 (21), 2 (14), 3 (25)
Rosette formation of hepatocytes
0 (20), 1 (28), 2 (12)
Focal necrosis
0 (0), 1 (5), 2 (20), 3 (12), 4 (23)
Stage
0 (22), 1 (11), 2 (10), 3 (6), 4 (0), 5 (6), 6 (5)
Table 1: Incidence of histological factors and stage in 60 AIH patients.
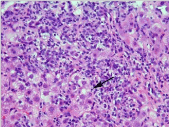
Figure 1: Interface hepatitis-related inflammation. Note interface hepatitis
associated with lymphoplasmacytic infiltrate and rosette formation of
hepatocytes (indicated by an arrow). HE stain.
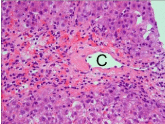
Figure 2: Centrilobular necrosis. Note hepatocellular necrosis around the
central vein (C). HE stain.
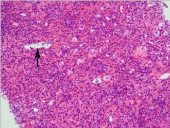
Figure 3: Pan-acinar necrosis. Note hepatocellular necrosis throughout the
entire acinus. The central vein is indicated by an arrow. HE stain.
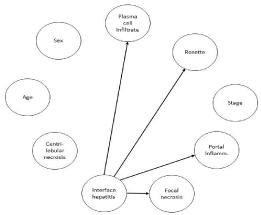
Figure 4: DAGs in BNs. Factors with relationships are indicated by arrows.
Interface hepatitis was related to plasma cell infiltrate, rosette formation of
hepatocytes, portal inflammation, and focal necrosis.
Discussion
AIH is an immune-mediated liver disease characterized by the presence of autoantibodies and high levels of gamma-globulin and histological evidence of portal-based hepatitis. Recently, the induction of AIH by autoreactive CD4+ T cells and effectors of γδT cells to liver damage in AIH were reported [11,13], but the mechanism of the inflammatory processes of AIH is not clearly understood.
The diagnosis of AIH was made based on the scoring system using clinical data and liver histology. The international AIH group reported a diagnostic system for AIH in 1999 [1]; thereafter, simplified criteria for the diagnosis of AIH were reported in 2008 [2]. The details and precise criteria were described in the former, and so in the current study, AIH patients, who were diagnosed using the former, were evaluated. In daily diagnosis based on liver biopsy, it frequently occurs that clinical data lead to suspected AIH, but liver histology shows other liver diseases, especially non-alcoholic steatohepatitis. Thus, the precise evaluation of liver histology is the most important factor for the diagnosis of AIH. Although CN was not included in the histological scoring system for the diagnosis of AIH, CN seemed to be a histological marker for the diagnosis of AIH in our previous studies of AIH [14,15].
The incidence of CN in AIH with acute presentation was reported as 31.7% [6], and the incidence of CN in the current study examining the classic type of AIH was 21.6%. These indicated that the incidence of CN in AIH may range from about 20-30%, even with the accumulation of cases with acute presentation. Recent studies proposed that the presence of CN without interface hepatitis should be diagnosed as the acute hepatitis phase of AIH [7]. For the evaluation of this proposal, we thought that the relationship between CN and typical inflammatory reactions of AIH should be studied, and we designed the present study. In this study, patients with AIH associated with interface hepatitis, i.e. the classic type of AIH, were selected and the relationship of CN with other histological factors including interface hepatitis was examined by the BNs, facilitating excellent statistical analysis for the examination of the relationships among various factors [8,9]. Subsequently, interface hepatitis, which was a base factor, was related to portal inflammation, plasmacytic infiltrate, rosette formation of hepatocytes, and focal necrosis. This relationship was termed as interface hepatitis-related inflammation. In this inflammation, lymphocytic infiltrate usually occurred, and so the possibility of the relationship of T cell-mediated inflammation with this inflammation was indicated. On the other hand, CN was not associated with interface hepatitis-related inflammation, and so the occurrence of CN caused by other mechanisms, such as ischemia, cardiac insufficiency, and drugs, was considered. Interestingly, patients with CN occasionally showed pan-acinar necrosis and frequently developed a clinically serious condition. In the present study, 4 among the 13 patients with CN had pan-acinar necrosis. In AIH, patients with pan-acinar necrosis are usually treated with corticosteroid administration; thus, CN in AIH may be a histological marker for the induction of corticosteroid treatment rather than a histologically diagnostic marker of AIH.
Conclusion
In conclusion, in AIH, interface hepatitis-related inflammation occurs, and CN occasionally overlaps. When CN progresses to panacinar necrosis, a clinically serious condition occurs in patients. CN is caused by a mechanism differing from interface hepatitis-related inflammation. The recognition of these is useful for the diagnosis and treatment of AIH.
Acknowledgement
The authors thank Mr. Mrozek (Medical English Service, Kyoto, Japan) for organizing English revision of this article.
References
- Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al., International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999; 31: 929-938.
- Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, et al., Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008; 48: 169-176.
- Te HS, Koukoulis G, Ganger DR. Autoimmune hepatitis: a histological variant associated with prominent centrilobular necrosis. Gut. 1997; 41: 269-271.
- Misdraji J, Thiim M, Graeme-Cook FM. Autoimmune hepatitis with centrilobular necrosis. Am J Surg Pathol. 2004; 28: 471-478.
- Zen Y, Notsumata K, Tanaka N, Nakanuma Y. Hepatic centrilobular zonal necrosis with positive antinuclear antibody: a unique subtype or early disease of autoimmune hepatitis? Hum Pathol. 2007; 38: 1669-1675.
- Abe K, Kanno Y, Okai K, Katsushima F, Monoe K, Saito H, et al., Centrilobular necrosis in acute presentation of Japanese patients with type 1 autoimmune hepatitis. World J Hepatol. 2012; 4: 262-267.
- Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol. 2006; 59: 246-249.
- Scutari M. Learning Bayesian networks with the bnlearn R package. J Stat Soft 2010; 35: 1-22.
- Scutari M, Denis J-B. Bayesian networks with examples in R. CRC Press, Taylor & Francis Group, Boca Raton, London, New York, 2015; 1-225.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F,et al., Histological grading and staging of chronic hepatitis. J Hepatol. 1995; 22: 696-699.
- Kido M, Watanabe N, Okazaki T, et al. Fatal autoimmune hepatitis induced by concurrent loss of naturally arising regulatory T cells and PD-1-mediated signaling. Gastroenterology 2008; 135: 1333-1343.
- Ferri S1, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, Hussain MJ . A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010; 52: 999-1007.
- Longhi MS, Hussain MJ, Kwok WW, et al. Autoantigen-specific regulatory T cells, a potential tool for immune-tolerance reconstitution in type-2 autoimmune hepatitis. Hepatology 2011; 53: 536-547.
- Matsumoto T, Kobayashi S, Shimizu H, et al. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver 2000; 20: 366-373.
- Matsumoto T, Morizane T, Aoki Y, et al. Autoimmune hepatitis in primary Sjögren’s syndrome: pathological study of the livers and labial salivary glands in 17 patients with primary Sjögren’s syndrome. Pathol Int 2005; 55: 70-76.