
Rapid Communication
J Hepat Res. 2015; 2(3): 1032.
Prevalence of Pre-Core and Basal Core Promoter Mutations in HbeAg Negative Patients from India
Vadwai V and Das BR*
Research and Development, SRL Limited, India
*Corresponding author: Das BR, Research and Development, SRL Limited, Primer Square Building, Plot No.1, Gaiwadi Industrial Estate, S.V. Road, Goregaon (W), Mumbai, India
Received: August 20, 2015; Accepted: December 30, 2015; Published: December 31, 2015
Abstract
Hepatitis B e Antigen (HBeAg), a translational product of the pre-Core (pre-C) and Basal Core Promoter (BCP) region of the Hepatitis B Virus (HBV), and its presence in the patient serum indicates active viral replication. Seroconversion of HBeAg indicates response to therapy and resolution of the disease; but emergence of mutations in the pre-C and BCP regions of the viral genome inhibits the production and secretion of HBeAg giving a false impression of favorable therapeutic response. Occurrence of different pre-C and BCP mutations are known to be associated with different HBV genotypes. Thus, we aimed to determine the prevalence of pre-C and BCP mutations in 42 HBeAg negative patients and correlate its presence with HBV genotype. Genotype D (76.1%, 32/42 patients) was found to be predominant followed by genotype A (23.9%, 10/42 patients). Sequence analysis revealed mutations A1762T/ G1764A and G1896A to occur at a higher frequency in genotype A (60%, 6/10) and genotype D (71.8%, 23/32) respectively. Thus, the examination of pre-C and BCP mutations would be helpful for prediction of the clinical outcome.
Keywords: HBV genotype; Pre-core mutations; Basal core promoter mutations; India
Introduction
Hepatitis B Virus (HBV) infection is a global health problem, with hepatitis B and C accounting for approximately 80% of all HBV infections and kills close to 1.4 million people every year. These patients are at risk to develop liver complications (and hepatocellular carcinoma) during their lifetime [1]. The goal of HBV treatment is to prevent progression of liver disease to cirrhosis, hepatic failure, or hepatocellular carcinoma; but its outcome is dependent on several factors including viral load, mutations and HBV genotype.
In the natural history of chronic HBV infection, Hepatitis B ‘e’ Antigen (HBeAg) represents active virus replication. Thus early response to therapy is determined by loss of HBeAg and Seroconversion to antibody against HbeAg known as “inactive HBV carrier state”. Inspite of Seroconversion, some patients continue to show elevated serum viral load levels; and this is mostly associated with mutations in the pre-core (pre-C) and Basal Core Promoter (BCP) regions of the HBV genome. Mutations in the BCP and pre-C regions affect the transcriptional and translational activity leading to the loss of HBeAg expression and secretion, without affecting the viral replication. Double mutation A1762T/G1764A (BCP region) and single nucleotide transition G1896A (pre-C region) are the most common mutations affecting the HBeAg secretion and are known to be in association with liver disease progression [2,3]. Recent studies have also reported G1899A, C1653T, T1753V, C1766T and T1768A mutations to be in association with severe liver complications including hepatocellular carcinoma [4].
Besides these mutations, heterogeneity in HBV genome has also been known to be predictive of disease severity and response to interferon treatment. Genotypes A and C harbor a higher prevalence of A1762T/G1764A mutation; while genotypes B and D have a higher frequency of G1896A mutations [5]. This could be indicative of variable degree of pathogenesis and disease severity within different individuals based on variations in HBV genome and mutations possessed.
Thus, in the present study, we sought to determine if any significant association exists between HBV genotype and prevalence of mutations within the BCP and pre-C mutations at nucleotides 1753, 1762, 1764, 1766, 1768, 1896 and 1899 along with other HBV viral load and patient characteristics.
Materials and Methods
Setting and patient specimens
The study was performed at a standalone diagnostic laboratory in Mumbai, evaluating a total of 42 consecutive HBeAg negative serum samples received between June, 2012 and July, 2015. Ethical approval and patient consent was not required for the study as specimens were processed as a part of the routine diagnostic testing.
HBV quantification
HBV DNA levels were determined using the automated COBAS® ampliprep/COBAS® TaqMan® HBV Test, v2.0 kit (Roche) as per manufacturer’s instructions.
HBV genotyping
DNA extraction was performed using the QIAamp Mini kit (Qiagen) as per recommended protocol. Amplification of the pre-S region conferring diverse heterogeneity and bidirectional sequencing was performed as per a previous published protocol [6]. The sequence obtained was analyzed using the NCBI blast program (https://blast.ncbi.nlm.nih.gov) to determine the viral genotype.
Mutational testing
Amplification and sequencing of the BCP and pre-C regions was done as published elsewhere [6], and the obtained sequence was compared with reference genome sequence (NCBI id NC_003977.1). The obtained sequence was screened for mutations known to be associated with severe liver disease at nucleotides 1753, 1762, 1764, 1766, 1768, 1896 and 1899.
Statistical analysis
One-way analysis of variance was conducted to compare mean quantitative values and the chi-square or Fischer’s exact test for categorical variables (SPSS ver.12). p-value <0.05 was considered as statistically significant.
Results
Patient characteristics and mutational prevalence
A total of 42 patients (33 [78.5%] males; 10 [21.5%] females) with a mean age of 39.1 years (±12 years, standard deviation [SD]) were included in the study. HBV genotyping results showed 32 (76.1%) patients infected with genotype D and 10 (23.9%) patients infected with genotype A; with a mean HIV viral load of log10 5.1 (±1.4, SD) and log10 4.8 (±1.1, SD) respectively.
In the BCP region, the double nucleotide mutation A1762T/ G1764A occurred frequently (50%, 21/42), followed by T1753V (30.9%, 13/42) and double mutations C1766T/T1768A (4.7%, 2/42) respectively. Similarly, in the pre-C region, mutation at nucleotide 1896 (27/42 patients, 57.1%) was found at a higher frequency than at nucleotide position 1899 (10/42 patients, 23.8%). Certain HBV strains harbored simultaneous mutations in both BCP and pre-C regions but none were found to be in association with any genotype, p>0.05 (Figure 1).
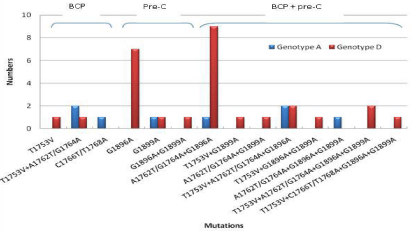
Figure 1: BCP and pre-C region mutational pattern among different HBV
genotypes.
Correlation of BCP and pre-C mutations with HBV genotype and serum DNA level
Double mutation A1762T/G1764A was found at higher frequency (p=0.6) in genotype A than genotype D, 60% (6/10) vs 46.8% (15/32); while mutations G1896A was found at a higher frequency (p=0.3) in genotype D, 71.8% (23/32) compared to genotype A, 40% (4/10). Also, multiple mutations were found at a higher frequency than single mutations, 59.5% (25/42) vs 26.1% (11/42) (p=0.03). HBV serum levels with respect to different mutations have been shown in (Figure 2).
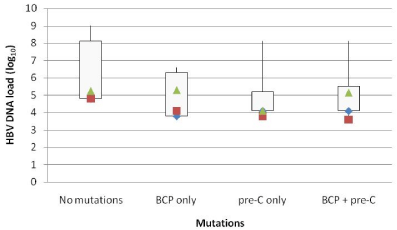
Figure 2: Box plot represents correlation of serum HBV DNA levels with
respect to different mutations observed in the BCP- and / or pre-C regions
of the HBV genome.
Discussion
In our study, majority of patients were infected with HBV genotype D (76.1%, 32/42) and only 23.9% (10/42) with genotype A; these results are similar to other previously reported studies in India [7- 9]. Genotype A has been known to achieve an earlier Seroconversion than genotype D; and is also known to have a higher frequency of BCP and pre-C mutations respectively [5]. In the present study, double mutation A1762T/G1764A was found at a higher frequency (p=0.11) in genotype D (71.5%, 15/21) compared to genotype A (28.5%, 6/21); a finding similar to that reported at different geographical locations in India [8,10]. The reason for the higher prevalence of A1762T/G1764A double mutation is unclear. Further analysis revealed G1896A mutation (either alone or in combination with other mutations) was the major cause of Seroconversion in 64.2% (27/42) patients (71.8% [23/32] patients were genotype D; 40% [4/10] patients were genotype A). This result was consistent with previous data showing that G1896A mutation was the strongest factor of HBeAg clearance in genotype D individuals. Similarly, high mutational prevalence compared to genotype A has been reported previously from North India (36.9- 52% vs 0-4.8%) [8,10].The basis of the relation between G1896A mutation and HBV genotypes is due to the base pairing between nt 1896 and nt 1858 in the stem loop structure of encapsidation signal which is essential for viral replication. Therefore, G1896A is restricted to genotypes that have T at nt 1858 but is known to occur rarely in genotypes that have C at nt 1858 [11]; which is similar to the findings of our study (data not shown) .
The co-existence of A1762T/G1764A and G1896A mutations was observed in 40.2% (17/42) patients. Besides these, other mutations at nucleotide positions 1753, 1766, 1768 and 1899 known to be involved in liver disease severity was also found to exist along with the common mutations, at a frequency lower compared to that reported by a study in Morocco (23% vs 64%) [4]. However the true mechanism for pathogenesis of these mutations remains unknown. An important finding of the study was that none of the mutations showed any association with HBV DNA serum levels, a finding in contrast to that reported by Kitab et al., [4].
In summary, although HBV genotype is known to influence liver disease severity; precise determination of BCP and pre-C mutations can help design better patient management strategies for follow-up and monitoring therapeutic outcome.
References
- World Health Organization. Hepatitis B virus Fact Sheet 2015.
- Kramvis A, Kew MC. Structure and function of the encapsidation signal of hepadnaviridae. J Viral Hepat. 1998; 5: 357-367.
- Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989; 2: 588-591.
- Kitab B, Essaid El Feydi A, Afifi R, Trepo C, Benazzouz M, Essamri W, et al. Variability in the precore and core promoter regions of HBV strains in Morocco: characterization and impact on liver disease progression. PLoS One. 2012; 7: e42891.
- Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: Recent advances. J Gastroenterol Hepatol. 2011; 26 Suppl 1: 123-130.
- Alfaresi M, Elkoush A, Alshehhi H, Alzaabi A, Islam A. Hepatitis B virus genotypes and precore and core mutants in UAE patients. Virol J. 2010; 7: 160.
- Banerjee A, Banerjee S, Chowdhury A, Santra A, Chowdhury S, Roychowdhury S, et al. Nucleic acid sequence analysis of basal core promoter/precore/ core region of hepatitis B virus isolated from chronic carriers of the virus from Kolkata, eastern India: low frequency of mutation in the precore region. Intervirology. 2005; 48: 389-399.
- Sharma S, Sharma B, Singla B, Chawla YK, Chakraborti A, Saini N, et al. Clinical significance of genotypes and precore/basal core promoter mutations in HBV related chronic liver disease patients in North India. Dig Dis Sci. 2010; 55: 794-802.
- Chauhan R, Kazim SN, Bhattacharjee J, Sakhuja P, Sarin SK. Basal core promoter, precore region mutations of HBV and their association with e antigen, genotype, and severity of liver disease in patients with chronic hepatitis B in India. J Med Virol. 2006; 78: 1047-1054.
- Agarwal AK, Sen S, Banerjee D, Srivastava R, Praharaj AK. Distribution of hepatitis B virus genotype and cancer predicting precore and basal core promoter mutations. Med J Armed Forces India. 2015; 71: 225-232.
- Kramvis A, Kew MC. Relationship of genotypes of hepatitis B virus to mutations, disease progression and response to antiviral therapy. J Viral Hepat. 2005; 12: 456-464.