
Research Article
J Hepat Res. 2021; 6(1): 1043.
Sialylation of HCV E2 Glycoprotein-Specific and Natural Anti-Glycan (TF, αGal) Antibodies as Signatures of Liver Damage
Kurtenkov O*, Jakovleva J, Sergejev B and Geller J
Department of Virology and Immunology, National Institute for Health Development, Estonia
*Corresponding author: Kurtenkov O, Department of Virology and Immunology, National Institute for Health Development, Hiiu 42, Tallinn 11619, Estonia
Received: May 05, 2021; Accepted: May 25, 2021; Published: June 01, 2021
Abstract
The E2 glycoprotein is the target of broadly neutralizing antibodies against Hepatitis C Virus (HCV). There is evidence that the HCV E2-specific antibody glycosylation profile is associated with hepatic fibrosis progression. The main aim of this study was to compare the sialylation of E2-specific and naturally occurring antiglycan Abs to determine whether their combination could be beneficial for the non-invasive evaluation of hepatic damage. Fifty-eight patients with various stages of hepatic fibrosis or without were tested. The sialylation of HCV E2 glycoprotein-specific antibodies (E2-Abs), the Thomsen-Friedenreich antigen- and αGal glycotope-specific antibodies (TF-Abs, αGal-Abs) was analysed using the ELISA platform. The level of IgG Abs and their reactivity to Sialospecific Sambucus Nigra Lectin (SNA) were determined and changes in Abs sialylation were analysed based on the stage of liver fibrosis, HCV genotype and antiviral therapy efficacy. The late stage of liver Fibrosis (F4) was characterized by dramatically decreased E2-Ab SNA reactivity unlike stages with no fibrosis (P=0.003) and stages F1–F3 (P=0.0007). In contrast, antiglycan Abs showed an increased sialylation. In multiple regression analysis, the combination of E2 and TF-Abs sialylation patterns gave a significant advantage in assessing liver damage. A high rate of discrimination between F0 and F4 stages of fibrosis as well as between F1–F3 and F4 was obtained (ACC=0.948 and ACC=0.90, respectively). Thus, the combined analysis of disease-specific and natural Abs sialylation can remarkably enhance the clinical value of the approach in the non-invasive evaluation of hepatic damage.
Keywords: HCV infection; Anti-E2 antibodies; αGal-specific antibodies; TF–specific antibodies; Antibody sialylation; Hepatic fibrosis
Abbreviations
Ab: Antibody (ies); ACC: Accuracy; AUC: The Area Under the Curve in ROC Analysis; E2-Ab: Antibody to HCV E2 Glycoprotein; E2-SNA: The Level of SNA Binding to E2-Specific Antibodies; ELISA: The Enzyme-Linked Immunosorbent Assay; Gal-Ab/IgG: αGal-Specific Antibodies/IgG; Gal-SNA: The Level of SNA Binding to Alpha-Gal Glycotope Specific Antibodies; IF-RBV: Pegylated Interferon-α-2a Plus Ribavirin Therapy; ACC: Accuracy of ROC analysis; ROC: Receiver Operator Curve Analysis; SNA: Sambucus Nigra Agglutinin; TF-Ab/IgG: TFantigen-Specific Antibodies/IgG; TF-SNA: The Level of SNA Binding to TF-Specific Antibodies; TFα: Thomsen-Friedenreich Antigen (Galα1-3Gal NAc-R, alpha anomer); αGal: Xenogenic Alpha-Gal Glycotope (Galα1-3Galβ1-4GlcNAc-R)
Introduction
The E2 glycoprotein (gp70) is one of the main targets of broadly neutralizing antibodies against hepatic C virus (HCV) [1-3]. Antibody glycosylation is a critical modification of antibody activity occurring via Fc-mediated effector mechanisms [4-8]. The diverse clinical effect of different Ab glyco-subsets has been demonstrated in autoimmunity, infections, and cancer [9-13]. We have shown recently that the sialylation of E2-specific Abs is associated with hepatic fibrosis progression [14]. Alterations in the glycosylation of immunoglobulins and some other glycoproteins (haptoglobin, α-fetoprotein, α1-antitripsin) have been described in patients with HCV infection [15-17].
Many human natural antibodies to glycans, including the socalled Thomsen-Friedenreich antigen (Galα1-3Gal NAc-R) and αGal glycotope (Galα1-3Galβ1-4GlcNAc-R), are produced mostly against carbohydrate antigens on gastrointestinal bacteria and may interact with microbial and viral envelope glycoproteins carrying these epitopes, thus protecting the human organism against bacteria and viruses [18-24]. The presence of anti-microbial Abs in patients with liver cirrhosis may reflect an increased bacterial translocation [25]. Abs against the Thomsen-Friedenreich (TFα) glycotope play an important role in the elimination of TF-expressing neoplastic cells [26], and a higher sialylation of anti-TF Abs was revealed in patients with cancer [27].
The increased galactosylation and fucosylation of αGal-specific IgG have been found to occur in the late stages of HCV-induced hepatic fibrosis [15]. Notably, compared with total serum IgG, these changes were mostly observed with anti-αGal IgG. The TF glycotopespecific Abs have not yet been investigated in this respect. Thus, there is evidence that the glycosylation of E2-specific and natural Abs to αGal glycotope is altered in the late stages of hepatic fibrosis induced by HCV infection. Whether these changes are common to both HCV-specific Abs and antibodies to HCV non-related targets remains yet unknown. The main aim of this study was to compare the sialylation profile of E2-specific and naturally occurring antiglycan Abs and determine whether their combination could contribute to the non-invasive evaluation of hepatic damage. The benefit of such combination is demonstrated and discussed.
Material and Methods
Subjects
Serum samples were taken from fifty-eight HCV infected treatment-naïve patients: men-32 and women-26 of median age of 37 years (range 19–57). The HCV genotype-based characteristics of patients and the effect of antiviral therapy are presented in Table 1. The investigation was carried out in accordance with the ICH GCP Standards and approved by Tallinn Medical Research Ethics Committee, Estonia. A written informed consent was obtained from all patients.
HCV
Hepatic fibrosis stage
Response to treatment
Genotype
0
1
2
3
4
SVR
NR
RL
ST
(n)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
n (%)
1b (26)
11 (42,3)
6 (23,1)
0 (0)
3 (11,5)
6 (23,1)
12 (46,2)
7 (26,9)
4 (15,4)
3 (11,5)
3a (27)
14 (51,9)
5 (18,5)
3 (11,1)
1 (3,7)
4 (14,8)
21 (77,8)
1 (3,7)
2 (7,4)
3 (11,1)
1a+2a/2c (5)
3 (60)
1 (20)
1 (20)
0 (0)
0 (0)
4 (80)
0 (0)
0 (0)
1 (20)
All cases (58)
28 (48,3)
12 (20,7)
4 (6,9)
4 (6,9)
10 (17,2)
37 (63,8)
8 (13,8)
6 (10,3)
7 (12,1)
Genotype 1a- one patient and GT 2a/2c–four patients. SVR: Sustained Virologic Response, NR: No Response, RL: Relapse. In seven patients treatment was Stopped (ST) due to intolerance.
Table 1: The characteristics of patients under investigation.
The diagnosis of HCV infection was based on the presence of anti-HCV antibodies in the patients sera, detection of serum HCV RNA, histologically verified fibrosis stage and clinical follow-up. The HCV genotype was determined by the hybridization technique using VERSANT HCV genotype assay (LiPA) (Bayer HealthCare LLC, Tarrytown, NY). The range of fibrosis was classified according to the Metavir scoring system from F0 to F4 (cirrhosis). The serum samples were stored in aliquots at -40ºC until use.
HCV infection treatment (pegylated interferon-α-2a plus ribavirin therapy) (IFN-RBV) was conducted according to the National Guidelines approved by the Estonian Society for Infectious Diseases in 2010. The response to therapy was evaluated as a Sustained Virological Response (SVR), No Response (NR) or Relapse (RL) at 24 weeks following the end of treatment.
The E2 glycoprotein- and glycan-specific IgG antibody assay
The level of anti-E2 and two antiglycan IgG antibodies was determined by the enzyme-linked immunosorbent assay (ELISA) as described earlier [14,28], with minor modifications. The plates (NUNC Maxisorp, Denmark) were coated with an E2 recombinant protein (ViroStat, ME, USA), or with a synthetic TFα-polyacrylamide conjugate (10%), and αGal-polyacrylamide conjugate (20%) (Lectinity, Russia) in carbonate buffer, pH 9.6, 2.5 μg/ml and 5 μg/ml for E2 and antiglycan Abs, respectively. After the overnight incubation, triple washing and blocking with a Superblock solution (Pierce, USA) for 30 min at 25ºC, the serum samples diluted 1:50 in PBS-0.05% Tween (Tw) were applied for 1.5 h at 25ºC. After the subsequent washing with PBS-Tw, the level of bound Abs was determined using the Alkaline Phosphatase (AP) conjugated goat anti-human IgG, (Sigma, USA) and p-nitrophenylphosphate disodium hexahydrate (pNPP, Sigma, USA). The absorbance values were read at 405 nm (Tecan Reader, Austria). The Optical Density Value (OD) of control wells (no serum sample) was subtracted from that of Abs-coated wells and each sample was analysed in duplicate.
SNA lectin reactivity of E2 and anti-glycan antibodies
The lectin reactivity of E2-specific and anti-glycan antibodies was measured in a similar way, except that the binding of the neuraminic acid (sialic acid)-specific Sambucus Nigra Agglutinin (SNA) to the absorbed antibodies was determined. After triple washing with the biotinylated SNA (Vector Laboratories, Inc., USA) in 10 mmol/L Hepes, 0.15 mol/L NaCl, 0.1 mmol/L CaCl2, pH 7.5 was applied at a concentration of 5 μg/mL for 1.5 h at 25ºC. The bound lectin was detected with a streptavidin-AP conjugate (Dako, USA) and pNPP (Sigma, USA). The O.D. of control wells (no serum sample) was subtracted from that of serum-coated wells to determine the lectin binding. Each sample was analysed in duplicate. The value of the SNA binding to Abs and the ratio of SNA binding to IgG level (SNA/IgG ratio) were determined. In addition, the ratio of E2-Ab SNA reactivity (E2-SNA) to TF-SNA or Gal-SNA values (E2-SNA/TF-SNA or E2- SNA/Gal-SNA) were calculated.
Statistical analysis
Comparisons between the groups were performed using the nonparametric Mann–Whitney U test for unpaired data or Student’s t-test where appropriate and P ≤ 0.05 value was considered statistically significant. The Receiver Operator Characteristic (ROC) curve analysis was used to evaluate the sensitivity and specificity of the changes found in hepatic fibrosis. The differences in Abs levels and SNA reactivity were also evaluated by multiple regression analysis using the combination of parameters for the diagnostic accuracy discrimination between hepatic fibrosis stages. All calculations were performed using the STATISTICA 10 and RStudio-1.1.463 software.
Results
The E2-Abs level was significantly higher in patients with stage F4 fibrosis than in those with stage F0, P=0.007. The level of both anti-glycan Abs was also increased in stage F4 of fibrosis (P=0.04). This was also true for αGal antibodies in stages F1–F3 vs F0 (P=0.01) (Figure 1(c)).
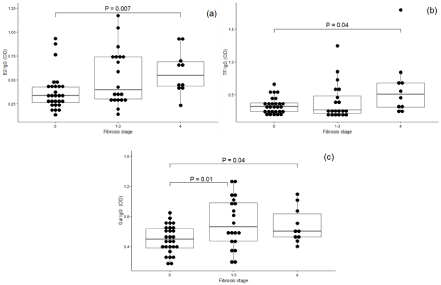
Figure 1: The level of anti-E2, TF and αGal IgG antibodies by hepatic fibrosis stage (F0–F4).
(a) Anti-E2 IgG level; (b) TFα and (c) αGal antibodies.
Each dot corresponds to one individual. Medians, ranges and quartiles are shown and P values are indicated for significant differences. Stages F1, F2 and F3 of
fibrosis are combined due to the small number of patients (n=12, 4 and 4, respectively).
In contrast, the SNA binding to E2-Abs (Figure 2) was much lower in F4 group than in groups F0 or F1-F3 (P=0.003 and P=0.00075, respectively), but was higher to TF-Abs (P=0.02, F0 vs F1–F3 and P=0.14 for F0 vs F4). A similar trend in increase was observed for Gal Abs (P=0.12; F0 vs F4). This regularity persists despite great interindividual variations. of antiglycan Abs levels independent of fibrosis stage.
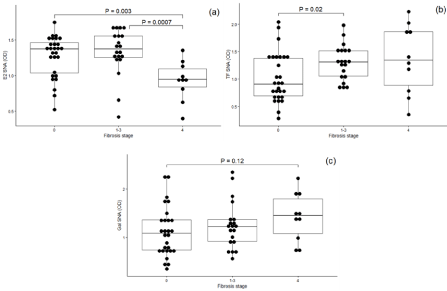
Figure 2: E2-specific, TF and αGal antibody SNA reactivity by hepatic fibrosis stage: (a) E2 IgG; (b) TFα (c) αGal. Medians, ranges and quartiles are shown and
P values are indicated for significant differences.
Both the E2-Abs SNA /TF Abs-SNA and E2-SNA/Gal-SNA ratios showed lower values in stage F4 than in stage F0 (P=0.007 and P=0.001, respectively) (Figure 3). Significant yet less pronounced changes were observed also for F0 vs F1–F3 fibrosis stages (P=0.04 and P=0.02, for TF-Abs and Gal-Abs, respectively). In patients with no fibrosis (F0 stage), a positive correlation was found to exist between the SNA binding to TF-Abs and αGal-Abs (r=0.604, P=0.0006). This was also true for fibrosis stage F4 (r=0.86, P=0.001), whereas there was no correlation between Abs levels. In stages F2–F3, no significant correlation between these levels was found.
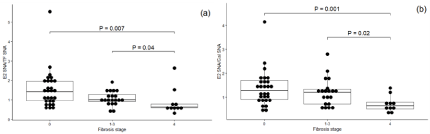
Figure 3: The ratio of E2 Abs SNA reactivity to TF Abs and αGal Abs SNA binding in patients with fibrosis stages F0, F1–F3 and F4.
(a) E2 SNA/TF SNA ratio; (b) E2 SNA/ αGal SNA ratio.
Given the ambiguos and partly opposite changes in E2-Abs and anti-glycan Abs sialylation all the tested parameters were analysed by multiple regression analysis. In discriminating fibrosis stages F0 and F4, the E2 Abs-SNA and TF Abs-SNA binding profile showed the ACC values of 0.789 and 0.71, respectively (Table 2, Figure 4 (a,b)).
Variables
E2-SNA (OD)
TF-SNA (OD)
E2-SNA + TF SNA
Predicted valuesAUC
0.8214
0.6571
0.9964
95% CI
0.6886-0.9542
0.4396-0.8747
0.9849-1.008
P Value
0.003
0.1
<0.0001
Cutoff
0.9585
1.39
0.1653
Sensitivity
0.8571
0.7857
0.9643
Specificity
0.6
0.5
0.9
ACC
0.7895
0.7105
0.9487
Table 2: The E2- and TF- specific antibody sialylation in discriminating F0 and F4 stages of hepatic fibrosis as determined/by ROC and multiple regression analysis.
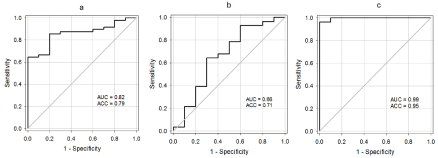
Figure 4: The diagnostic accuracy of discrimination between F0 and F4 fibrosis stages by ROC (The term is given above). Using E2- and TF-specific antibody
sialylation profiles: (a) E2-Abs; (b) TF-Abs; (c) a combination of E2 and TF antibodies as determined by multiple regression statistics. Predicted values calculated
by multiple regression analysis: Z = –0.47808* (X E2-SNA (OD)) + 0.307414* (X TF-SNA (OD)) + 0.754585.
The highest ability of discrimination (ACC=0.948) between fibrosis stages F0 and F4 was obtained using a combination of two parameters, E2-SNA and TF-SNA. Notably, a high discrimination ability persisted also in comparing stages F1–F3 with F4: the area under curve 0.905, sensitivity 0.85, specificity 1.0, ACC 0.90). The combination of E2-SNA and Gal-SNA parameters showed a lower accuracy of the assay (ACC=0.833).
Patients with HCV 1b genotype demonstrated a clear trend to higher E2 Abs levels compared with GT3 (P=0.054) but antiglycan Abs levels remained unchanged, differently from other genotypes. No significant differences in SNA binding was found, except a higher E2- SNA/IgG ratio (P=0.015) in patients infected with HCV 3a genotype, unlike GT1b. The HCV genotype was not associated with changes of TF-Abs and Gal-Abs parameters (data not shown).
E2, TFα and αGal specific Abs levels showed no relationship with the efficacy of RBV-IF virotherapy. A higher level of SNA binding to αGal Abs was found in patients with relapse (RL) compared with non-responders (significant for Gal, P=0.03) and the SVR group (P=0.005) (Figure 5). A similar trend in TF Abs levels (P=0.10) was observed in RL and NR groups. The “Stop Treatment” (ST) group showed a profile similar to “no response” patients.
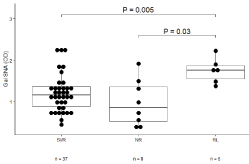
Figure 5: The SNA binding to αGal-specific antibodies and IFN-RBV therapy
efficacy.
Abbreviations: SVR: Sustained Virologic Response; NR: No Response;
RL: Relapse. Medians, ranges and quartiles are shown and P values are
indicated for significant differences.
Thus, based on the predicted values calculated by multiple regression analysis of the combination of two parameters (E2-SNA and TF-SNA), hepatic cirrhosis (F4 stage of fibrosis) may be present with high accuracy distinctive of other stages of hepatic damage, or be absent (stage F0). It appears that an increased sialylation of TFand Gal-Abs is more often observed in patients with a future relapse, which is predictive of IF-RBV virotherapy inefficacy.
Discussion
The important role of anti-envelope Abs response in the resistance to and a spontaneous cure of HCV infection has been demonstrated [1-3,29] but E2-Abs structural heterogeneity, in particular, the glycosylation profile remains mostly unexplored, yet. Our recent findings showed a significant decrease in E2-Abs sialylation in the late stages of hepatic fibrosis [14]. It is considered that a broad spectrum of naturally occurring antiglycan Abs in humans are induced by enteric microorganisms or their products [30,31]. Significant alterations of intestinal microbiota with microbial translocation and hepatic inflammation occur in the late stages of fibrosis [30,32]. However, there is still no evidence that TF- or Galepitope expressing microbiota takes part in such translocation. No information exists about the possible expression of TFα or αGal glycotopes in hepatocytes or stromal cells during HCV infection either.
Two naturally occurring Abs specific to TFα and αGal glycotopes were included in this study due to their natural ubiquitous presence in each individual, being thus convenient glycotope-specific targets in contrast to highly polymorphic total serum IgG (tIgG). Approximately 1% of human blood immunoglobulins belong to Abs against xenogeneic αGal epitope [24, 33]. A much lower amount of TF specific Abs is present in human serum: about 0.5 mkg and 5 mkg per ml for IgG and IgM, respectively [34].
It is of interest that E2 and TF-Abs sialylation demonstrated quite opposite (and partly independent) changes in fibrosis stage F4, i.e. the decrease and increase, respectively. Therefore we assumed that the combination of these two parameters might be clinically more informative. In fact, the multiple regression statistics showed an appreciable advantage of such a combination compared with the sialylation analysis of single parameters alone. Another combination of parameters under study exhibited lower ACC values.
The reasons for changes in the sialylation of anti-E2 and anti-glycan Abs observed in this study remain yet unclear. It is well accepted that sialylated Igs have anti-inflammatory activity [4,13,35,36]. Alterations of Abs glycosylation in autoimmunity is a well-documented fact and the low level of IgG sialylation is usually associated with increased inflammation [6,7,10,16]. The agalactosylation and decreased sialylation of IgG appear as a proinflammatory mechanism common to numerous inflammatory diseases. It appears that the decreased sialylation of IgG is a proinflammatory mechanism common to numerous inflammatory diseases.
The most intriguing finding of the study was that changes in the sialylation of anti-E2 and anti-glycan Abs revealed quite opposite trends in the late stages of fibrosis, i.e. the decrease and increase, respectively, thus suggesting that both parameters are independent variables. Moreover, namely the sialylation changes of E2 and antiglycan Abs not their levels demonstrated an association with liver damage and therapy outcome. It is important to note that the total serum IgG glycosylation profile may significantly differ from that of antigen-specific IgG Abs involved in the pathogenesis of a specific disease [10,12,37-39], suggesting the presence of disease-specific IgG changes of potential clinical importance. This also implies that the glycosylation pattern of Abs against the target antigens involved in the pathogenesis of a specific disease may be more informative than just the level of IgG Abs to a specific antigen. It seems that a decreased sialylation of the HCV specific E2 Abs in the late stages of fibrosis may reflect a higher degree of inflammation due to the proinflammatory activity of hyposialylated antibodies and represent an epiphenomenon derived from the inflammatory environment induced by HCV.
We suggest that the higher proportion of SVR responders in patients with increased SNA binding to all tested Abs (especially αGal-Abs) compared with “no responders” could be explained by the anti-inflammatory activity of higher sialylated Abs. The decrease of E2-Abs sialylation could partly explain the observed association of this phenomenon with a higher hepatic damage as well as with a higher effect of virotherapy in these patients.
In conclusion, the significant and in part opposite changes in the sialylation of E2 and glycan-specific Abs were found in the late (F4) stage of hepatic fibrosis. As established by multiple regression analysis, the combination of E2-Abs and TF-Abs sialylation patterns provided a significant advantage in assessing liver damage with high accuracy (ACC higher than 90%). We suggest that the combined analysis of HCV-specific and natural anti-glycan Abs glycodiversity may substantially enhance the clinical value of the approach in the non-invasive evaluation of hepatic damage induced by HCV infection. Detailed retrospective and prospective analysis of E2 and antiglycan Abs glycosylation signatures as well as their association with inflammatory parameters will be needed to further assess their efficacy in clinical settings.
Acknowledgment
The authors are grateful to Dr. V. Brjalin for providing blood samples and clinical data, to Dr. E. Jõeste for the histological assessment of the liver biopsy, and R. Syld for the final correction of the English language.
This study was supported by the Estonian Science Foundation (grant no. 7650) and partly by VISBY projects no. 00747/2010 and no.00885/2011, and project IUT-42-1 (Ministry of Education and Research, Estonia).
Data Availability
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors have approved this version of the manuscript and declare that there is neither conflict of interest nor financial gain regarding the publication of this paper.
Authors’ Contributions
OK designed the research, analyzed and interpreted the data, wrote and finalized the manuscript. JJ performed the research, statistical analysis, constructed the figures and revised the manuscript. BS performed statistical ROC analysis and multiple regression analysis, JG analyzed and interpreted the data and revised the manuscript.
References
- Pestka JM, Zeisel MB, Bläser E, Schürmann P, Bartosch B, Cosset FL, et al. Rapid induction of virus-neutralizing antibodies and viral clearance in a single source outbreak of hepatitis C. Proceedings of the National Academy of Sciences of the United States of America. 2007; 104: 6025–6030.
- Swann RE, Mandalou P, Robinson MW, Ow MM, Foung SK, McLauchlan J, et al. Anti-envelope antibody responses in individuals at high risk of hepatitis C virus who resist infection. J Viral Hepat. 2016; 23: 873–880.
- Eliyahu S, Sharabi O, Elmedvi S, Timor R, Davidovich A, Vigneault F, et al. Antibody Repertoire Analysis of Hepatitis C Virus Infections Identifies Immune Signatures Associated With Spontaneous Clearance. Front Immunol. 2018; 9: 3004.
- Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006; 313: 670–673.
- Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Current Opinion in Immunology. 2008; 20: 471–478.
- Böhm S, Schwab I, Lux A, Nimmerjahn F. The role of sialic acid as a modulator of the anti-inflammatory activity of IgG. Seminars in Immunopathology. 2012; 34: 443–453.
- Plomp R, Ruhaak LR, Uh HW, Reiding KR, Selman M, Houwing-Duistermaat JJ, et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Scientific Reports. 2017; 7: 12325.
- Seeling M, Brückner C, Nimmerjahn F. Differential antibody glycosylation in autoimmunity: sweet biomarker or modulator of disease activity? Nat Rev Rheumatol. 2017; 13: 621–630.
- Kodar K, Stadlmann J, Klaamas K, Sergeyev B, Kurtenkov O. Immunoglobulin G Fc N-glycan profiling in patients with gastric cancer by LC-ESI-MS: relation to tumor progression and survival. Glycoconj J. 2012; J29: 57–66.
- Shade KT, Anthony R. Antibody glycosylation and inflammation. Antibodies. 2013; 2: 392–414.
- Kurtenkov O, Izotova J, Klaamas K, Sergeyev B. Increased sialylation of anti- Thomsen-Friedenreich antigen (CD176) antibodies in patients with gastric cancer: a diagnostic and prognostic potential. Biomed Res Int. 2014; 830847.
- Alter G, Ottenhoff THM, Joosten SA. Antibody glycosylation in inflammation, disease and vaccination. Seminars in Immunology. 2018; 39: 102–110.
- Bartsch YC, Rahmöller J, Mertes MMM, Eiglmeier S, Lorenz FKM, Stoehr AD, et al. Sialylated autoantigen-reactive IgG antibodies attenuate disease development in autoimmune mouse models of lupus nephritis and rheumatoid arthritis. Frontiers in Immunology. 2018; 9: 1183.
- Kurtenkov O, Jakovleva J, Sergejev B, Geller J. Association of the Sialylation of Antibodies Specific to the HCV E2 Envelope Glycoprotein with Hepatic Fibrosis Progression and Antiviral Therapy Efficacy. Dis Markers. 2020; 8881279.
- Mehta AS, Long RE, Comunale MA, Wang M, Rodemich L, Krakover J, et al. Increased levels of galactose-deficient anti-Gal immunoglobulin G in the sera of hepatitis C virus-infected individuals with fibrosis and cirrhosis. Journal of Virology. 2008; 82: 1259–1270.
- Klein A, Carre Y, Louvet A, Michalski JC, Morelle W. Immunoglobulins are the major glycoproteins involved in the modifications of total serum N-glycome in cirrhotic patients. Proteomics Clin Appl. 2010: 4: 379–393.
- Comunale MA, Rodemich-Betesh L, Hafner J, Wang M, Norton P, Di Bisceglie AM, et al. Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients: implications for a biomarker of hepatocellular carcinoma. PLoS One. 2010; 5.
- Wiener AS. Origin of naturally occurring hemagglutinins and hemolysins; a review. J Immunol. 1951; 66: 287–295.
- Springer GF, Tegtmeyer H. Origin of anti-ThomsenFriedenreich (T) and Tn agglutinins in man and in white Leghorn chicks. British Journal of Haematology. 1981; 47: 453–460.
- Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural antialpha-galactosyl immunoglobulin G and bacteria of the human flora. Infection and Immunity. 1988; 56: 1730–1737.
- Klaamas K, Kurtenkov O, Rittenhouse-Olson K, Brjalin V, Miljukina L, Shljapnikova L, et al. Expression of tumor-associated Thomsen-Friedenreich antigen (T Ag) in Helicobacter pylori and modulation of T Ag specific immune response in infected individuals. Immunological Investigations. 2002; 31: 191–204.
- Henderson G, Ulsemer P, Schöber U, Löffler A, Alpert CA, Zimmermann- Kordmann M. Occurrence of the human tumor-specific antigen structure Galβ1-3GalNAcα- (Thomsen-Friedenreich) and related structures on gut bacteria: prevalence, immunochemical analysis and structural confirmation. Glycobiology. 2011; 21: 1277–1289.
- Ulsemer P, Toutounian K, Kressel G, Goletz C, Schmidt J, Karsten U, et al. Impact of oral consumption of heat-treated Bacteroides xylanisolvens DSM 23964 on the level of natural TFα-specific antibodies in human adults. Beneficial Microbes. 2016; 7: 485–500.
- Galili U. Host Synthesized Carbohydrate Antigens on Viral Glycoproteins as “Achilles’ Heel” of Viruses Contributing to Anti-Viral Immune Protection. Int J Mol Sci. 2020; 21: 6702.
- Papp M, Norman GL, Vitalis Z, Tornai I, Altorjay I, Foldi I, et al. Presence of antimicrobial antibodies in liver cirrhosis–a tell-tale sign of compromised immunity? PLoS One. 2010; 5: e12957.
- Springer GF, Desai PR, Ghazizadeh M, Tegtmeyer H. T/Tn pancarcinoma autoantigens: fundamental, diagnostic, and prognostic aspects. Cancer Detection and Prevention. 1995; 19: 173–182.
- Kurtenkov O. Profiling of Naturally Occurring Antibodies to the Thomsen- Friedenreich Antigen in Health and Cancer: The Diversity and Clinical Potential. Biomed Res Int. 2020; 9747040.
- Kurtenkov O, Klaamas K. Hidden IgG Antibodies to the Tumor-Associated Thomsen-Friedenreich Antigen in Gastric Cancer Patients: Lectin Reactivity, Avidity, and Clinical Relevance. Biomed Res Int. 2017; 6097647.
- Olbrich A, Wardemann H, Böhm S, Rother K, Colpitts CC, Wrensch F, et al. Erratum to: Repertoire and Neutralizing Activity of Antibodies Against Hepatitis C Virus E2 Peptide in Patients With Spontaneous Resolution of Hepatitis C. J Infect Dis. 2020; J221: 2084.
- Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. Journal of Hepatology. 2014; 60: 197–209.
- Schneider C, Smith DF, Cummings RD, Boligan KF, Hamilton RG, Bochner BS, et al. The human IgG anti-carbohydrate repertoire exhibits a universal architecture and contains specificity for microbial attachment sites. Science Translational Medicine. 2015; 7.
- Sandler NG, Koh C, Roque A, Eccleston JL, Siegel RB, Demino M, et al. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011; 141: 1220–1230.
- Galili U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefiits. Immunology. 2013; 140: 1–11.
- Butschak G, Karsten U. Isolation and characterization of thomsenfriedenreich- specific antibodies from human seerum. Tumour Biol. 2002; 23: 113–122.
- Maverakis E, Kim K, Shimoda M, Gershwin ME, Patel F, Wilken R, et al. Glycans in the immune system and The Altered Glycan Theory of Autoimmunity: a critical review. J Autoimmun. 2015; 57: 1–13.
- Markina YV, Gerasimova EV, Markin AM, Glanz VY, Wu WK, Sobenin IA, et al. Sialylated Immunoglobulins for the Treatment of Immuno-Inflammatory Diseases. Int J Mol Sci. 2020; 21: 5472.
- Xu PC, Gou SJ, Yang XW, Cui Z, Jia XY, Chen M, et al. Influence of variable domain glycosylation on anti-neutrophil cytoplasmic autoantibodies and antiglomerular basement membrane autoantibodies. BMC Immunol. 2012; 13.
- Zhu J, Lin Z, Wu J, Yin H, Dai J, Feng Z, et al. Analysis of serum haptoglobin fucosylation in hepatocellular carcinoma and liver cirrhosis of different etiologies. J Proteome Res. 2014; 13: 2986–2997.
- Mondal G, Saroha A, Bose PP, Chatterjee BP. Altered glycosylation, expression of serum haptoglobin and alpha-1-antitrypsin in chronic hepatitis C, hepatitis C induced liver cirrhosis and hepatocellular carcinoma patients. Glycoconj J. 2016; 33: 209–218.