
Research Article
J Hepat Res. 2021; 6(1): 1044.
Ribavirin Therapy in Immunodeficient Patients with Chronic Hepatitis E Virus Infection
Materacki L¹*, Valliani T¹, Gordon FH²
¹North Bristol Liver Unit, Southmead Hospital, Southmead Road, Bristol, UK
²Department of Liver Medicine, Bristol Royal Infirmary, Upper Maudlin St, Bristol, UK
*Corresponding author: Luke Materacki, North Bristol Liver Unit, Southmead Hospital, Southmead Road, Bristol, UK
Received: May 28, 2021; Accepted: June 25, 2021; Published: July 02, 2021
Abstract
Hepatitis E virus (HEV) infection, usually self-limiting in immunocompetent individuals, may adopt a chronic course in immunodeficient patients. A low threshold for HEV screening in immunodeficient patients is advocated so early management can be initiated to minimise liver injury.
This retrospective, observational study evaluated all cases (n=11) of chronic HEV diagnosed in immunodeficient patients in 2 university hospitals in Bristol, UK between February 2014 and October 2017. We report our experiences in the management of chronic HEV including ribavirin use.
No patients achieved viral clearance spontaneously or with reduction of immunosuppression and so all were treated with ribavirin. The median time between chronic HEV diagnosis and initiation of ribavirin was 91 days (range, 1–293 days). The median ribavirin dose at initiation was 1000 mg/day (range 800-1200 mg/day) and at cessation was 900 mg/day (range 600–1200 mg/day), reduced due to anaemia.
Different end points guided treatment cessation including negative HEV RNA PCR serology +/- negative stool HEV RNA PCR +/- biochemical remission at various time points. Following ribavirin therapy, HEV relapse occurred in 1 patient. The mean duration of ribavirin therapy, excluding the patient who was re-treated, was 4.6 months. One significant adverse event of severe anaemia requiring transfusion was observed.
This case series supports the use of ribavirin monotherapy for chronic HEV in immunodeficient patients. Anaemia commonly developed prompting ribavirin dose reduction. We advocate 2 consecutive negative serum and stool HEV RNA PCR results sampled at 4 weekly intervals to guide ribavirin cessation.
Keywords: Hepatitis E; Hepatitis; Ribavirin
Abbreviations
HEV: Hepatitis E Virus; SOT: Solid Organ Transplant; EASL: European Association for the Study of the Liver; HIV: Human Immunodeficiency Virus; RNA: Ribonucleic Acid; SVR: Spontaneous Virological Response; NHS: National Health Service; CKD: Chronic Kidney Disease; RR: Reference Range; ALT: Alanine Aminotransferase; PCR: Polymerase Chain Reaction; ARFI: Acoustic Radiation Force Impulse
Introduction
HEV is the most common cause of acute viral hepatitis in the UK. Although infection is usually self-limiting, immunosuppressed patients may fail to eradicate the virus leading to chronic infection [1]. This may induce accelerated liver fibrosis, leading to cirrhosis in some patients [2]. Prompt diagnosis and treatment of chronic HEV is important as liver fibrosis may regress after viral clearance.
A large multi-centre retrospective study of SOT recipients with chronic HEV found cirrhosis developed in 10% [3]. No patients were identified in this study who spontaneously cleared the virus between 3 and 6 months after infection. Subsequent EASL clinical practice guidelines have recommended that chronic infection be defined as patients with persistent viraemia for more than 3 months, for the purposes of treatment [4].
HEV infection may manifest with subtle or no symptoms making diagnosis challenging. The clinical presentation of chronic HEV infection is similar in patients with haematological disorders, HIV, rheumatological conditions receiving heavy immunosuppression and SOT recipients [4]. Most patients will be asymptomatic but coryzal symptoms, tiredness, malaise and fever may be reported and physicians should exercise a low threshold for testing liver biochemistry, including a viral liver screen, if any symptoms are identified, particularly in immunocompromised patients. Similar practice should be adopted if liver dysfunction is detected during monitoring in asymptomatic patients taking immunosuppression.
Following HEV exposure, 50% to 67% of SOT recipients will develop chronic HEV hepatitis. This risk is increased by a low platelet count at the time of diagnosis of infection and the use of tacrolimusbased immunosuppressive therapy (rather than cyclosporine A) [2]. Reducing immunosuppressive therapy achieves sustained viral clearance in 30% of SOT recipients [4]. Those SOT recipients who spontaneously achieved viral clearance had a lower tacrolimus level and a lower daily steroid dose compared to those who remained viraemic [4]. If HEV clearance is not spontaneously achieved by reducing immunosuppressive therapy, EASL recommend a 3 month course of ribavirin monotherapy, extended to 6 months if HEV RNA remains detectable or if there is relapse after ceasing ribavirin [4]. Second-line therapy with pegylated interferon for 3 months can be considered in liver transplant patients who fail ribavirin.
Predicting which patients with chronic HEV who are less likely to achieve SVR with conventional therapy is desirable so a more tailored treatment regimen can be instigated early. The optimal ribavirin dose and duration of therapy to treat chronic HEV remains unanswered. We report a combined case series from 2 hospitals in Bristol, United Kingdom of immunocompromised patients with chronic HEV who were treated with ribavirin.
Materials and Methods
This retrospective, observational study evaluated all cases of chronic HEV diagnosed with ribavirin monotherapy in North Bristol NHS Trust and University Hospitals Bristol and Weston NHS Trust between February 2014 and October 2017 (11 patients). Chronic HEV infection was defined by the presence of persistent HEV RNA detectable in the serum for at least 3 months before treatment was commenced using analysis of frozen serum if necessary. All samples were analysed by quantitative PCR at the UK reference centre for HEV in Colindale, London. Clinical data was collected from patients’ written and electronic medical records. SVR was defined as an undetectable level of HEV RNA in the serum and stool at least 6 months post-ribavirin therapy.
Results
Patient characteristics (Table 1)
Variable
Value
Age-year (range)
Median
45 (21-79)
Sex-no. (%)
Male
6 (55)
Female
5 (45)
Immunosuppression at time of ribavirin initiation-no. (%)
Glucocorticoid
10 (83)
Tacrolimus
9 (75)
Mycophenolate Mofetil
3 (25)
Methotrexate
1 (8)
Azathioprine
1 (8)
Rituximab
1 (8)
Sirolimus
1 (8)
Interval between diagnosis of chronic HEV and ribavirin initiation – days (range)
Median
91 (1-293)
Indication to cease treatment-no. (%)
2 x negative serum and stool RNA PCR 4 weeks apart
6 (50)
2 x negative serum RNA PCR 4 weeks apart
4 (33)
1 x negative serum RNA PCR
1 (8)
Normal ALT
1 (8)
Duration of successful course of therapy-no. (%)
3 months
3 (27)
3.5 months
3 (27)
5 months
2 (18)
6 months
1 (9)
10.5 months
1 (9)
14 months (re-treatment course for relapse)
1 (9)
Sustained virological response-no. (%)
Negative stool and serum RNA PCR 6 months
6 (55)
Negative serum RNA PCR 6 months
2 (18)
Negative serum RNA PCR 6 months, stool RNA PCR 3 months
1 (9)
Negative serum RNA PCR 3 months
1 (9)
Normal ALT 6 months
1 (9)
Table 1: Demographic, clinical and treatment characteristics of the patients.
11 patients were identified with chronic HEV infection during the study period, 55% of whom were male, with an age range 21-79 y at diagnosis. All of these patients were immunosuppressed, due to either long term drug therapy (10 patients) or haematological malignancyrelated immune paresis. The HEV genotype was not routinely measured for our patients.
82% of patients were SOT recipients (renal (n=7), liver (n=2)). The median time from receiving a SOT to chronic HEV diagnosis was 64 months (range 12-168 months). One patient with neuro-sarcoidosis was receiving immunosuppressive therapy with methotrexate and prednisolone.
At the point of diagnosis of chronic HEV, 91% (n=10) of patients were medicated with at least 2 immunosuppressants. Immunosuppressant therapy included tacrolimus (n=9) prednisolone (n=10), mycophenolate mofetil (n=2), azathioprine (n=2), methotrexate (n=1), rituximab (n=1) and sirolimus (n=1). Prior to ribavirin treatment, patients’ trough tacrolimus level was 6.5 μgL-1 with a median of 6 μgL-1 and a range of 1.6-8.4 μgL-1.
Only the 2 liver transplant patients had a history of prior intrinsic liver disease (biliary atresia and autoimmune hepatitis). 91% (n=10) had pre-existing renal disease (80% CKD 2, 20% CKD 3). 7 of these 10 patients were renal transplant recipients and 2 were liver transplant recipients. There were no reported extra-hepatic manifestations of HEV infection.
When chronic HEV was diagnosed, 73% of patients (n=8) had an asymptomatic transaminitis and 27% (n=3) had clinical symptoms alongside a biochemical hepatitis such as abdominal pain, lethargy, weakness or diarrhoea.
Prior to treatment, the mean lymphocyte count was 1.68 109L-1 (range 0.53-3.95 109L-1, RR 1.00-4.00 109L-1), mean platelet count 256 109L-1 (range 138-355 109L-1, RR 150-400 109L-1), and mean ALT 112 UL-1 (range 18-219 UL-1, RR 10-60 UL-1).
Treatment
11 patients received a total of 12 courses of ribavirin treatment, since viral relapse occurred in 1 patient necessitating re-treatment.
The median time between the diagnosis of chronic HEV infection and the initiation of the first course of ribavirin therapy was 91 days (range 1-293 days). At the time of treatment initiation, 7 patients had detectable serum HEV RNA for at least 6 months but 4 patients had been infected for longer than 6 months. The serum HEV RNA was checked in all patients immediately before treatment and an additional baseline stool HEV RNA PCR was checked in 6 of 11 patients.
The main reason for the delay in the initiation of therapy from the point of diagnosis of chronic HEV was to monitor for spontaneous clearance, which did not occur in any of the patients. Three of the 10 patients who were immunosuppressed had their immunosuppressant therapy reduced (tacrolimus) or an agent ceased (sirolimus/ azathioprine) but this was unsuccessful in each case.
Ribavirin treatment was initially commenced at a median daily dose of 1000 mg, with a range of 800-1200 mg. The starting doses were prescribed based on body weight and all the patients were treatment naïve.
Criteria for stopping treatment
EASL 2018 guidance4 recommends assessment of HEV RNA in the serum and stool after 12 weeks of treatment and if the RNA is undetectable in both, then to stop the ribavirin. Most of these patients were treated before the publication of this guideline and so the indications to cease therapy varied, however all patients were treated for a minimum of 3 months. In all but 2 courses of treatment, 2 consecutive negative HEV RNA tests, tested 4 weeks apart, were required to stop treatment.
In 5 patients a negative stool and serum HEV RNA result 4 weeks apart was used as an indication to cease therapy; in 4 patients, 2 negative serum HEV RNA PCR results 4 weeks apart was used; normalisation of the ALT was used in 1 patient and 1 patient’s therapy was stopped due to haemolytic anaemia in the context of 1 negative serum HEV RNA sample after 3 months of treatment-this was the only patient in whom the virus recurred (Table 1).
The patient with HEV recurrence had neuro-sarcoidosis and was being treated with methotrexate when his ribavirin was stopped. Seven months after cessation he developed biochemical hepatitis and was found to have positive HEV RNA in his serum, stool and cerebrospinal fluid. The methotrexate was switched to mycophenolate mofetil with no subsequent evidence of viral clearance and so he was recommenced on ribavirin. 14 months later, once he had 2 consecutive stool and serum samples negative for HEV RNA 4 weeks apart, his ribavirin was stopped. During this period, his stool HEV RNA was intermittently negative.
The mean ribavirin treatment duration, including the second course of therapy for 1 patient (n=12), was 5.3 months with a median of 3.5 months and a range of 3-14 months. 10 of 12 courses of ribavirin lasted for 3-6 months, 1 course lasted 6-12 months and 1 lasted longer than 12 months (Table 1). The mean treatment duration of the successful courses was 5 months with a median of 3.5 months and a range of 3-14 months.
The main treatment duration (4.67 months) was shorter in the 3 patients who had their immunosuppression reduced pre-treatment compared to those who did not (6.2 months).
Adverse events
Anaemia complicated ribavirin therapy in 92% (n=11) of courses, requiring dose reduction in 5 patients and 1 patient needed a blood transfusion (4 units) during the course of treatment. The median daily dosage of ribavirin was 900 mg with a range of 600-1200 mg.
One patient experienced nausea and fatigue was documented during 4 courses of therapy.
Treatment outcome (Table 1)
SVR was confirmed by undetectable HEV RNA in both stool and serum at 6 months post treatment completion in 6 of the 11 patients; 3 of 11 had a negative serum HEV RNA PCR 6 months after the end of treatment, 1 of whom had a negative stool HEV RNA PCR 3 months after the end of treatment; 1 patient had a negative serum HEV RNA PCR 3 months after the end of treatment and 1 patient had not had any HEV testing after the end of treatment due to non-attendance but the ALT remains in the normal range.
The mean ALT, 6 months after the end of treatment, was 17.6 UL-1 (range 8-29 UL-1).
Evaluation of liver fibrosis
6 of the 11 patients had a pre-treatment FibroscanTM as a noninvasive assessment of fibrosis, which showed the absence of cirrhosis in all but 1 patient, whose scan was difficult to evaluate due to polycystic liver disease (score of 15.3 kPa). The median FibroscanTM score of the other 5 patients was 5.2 kPa with a range of 2.5-6.3 kPa.
2 further patients had undergone liver biopsy to investigate abnormal liver blood tests prior to screening for chronic HEV. Both these biopsies took place in July 2013 and showed an idiopathic chronic hepatitis but no evidence of fibrosis.
10 out of 11 patients had a FibroscanTM during therapy with ribavirin, which showed no fibrosis in 6 patients (<7kPA). One patient had a reading of 7.6 kPA but did not attend a follow up scan. One patient had a reading of 7.2 kPA which normalised to 5.7 kPA post treatment. Two patients had readings of above 10 kPA; 1 had a post treatment transient elastography by ARFI scan which was normal (5.57 kPa, normal <5.7 kPa) and the other had a follow-up ARFI scan showing a stiffness of 9.1 kPa. This patient has polycystic liver disease which was thought to be the reason for the elevated readings. No patients had evidence of cirrhosis by transient elastography assessment after completion of ribavirin therapy.
Discussion
This relatively small retrospective case series contributes to the growing experience of the diagnosis and management of chronic HEV infection in immunosuppressed patients. In particular, we considered the utility of a 3 month monitoring period post diagnosis (to define chronic infection) and looked at the effect on treatment outcome of different stopping criteria. We also sought to describe further the pattern of chronic HEV infection in this group.
The majority of patients observed with chronic HEV presented as an asymptomatic transaminitis highlighting the importance of vigilant monitoring in patients receiving immunosuppression. Patients should be counselled that they cannot be reassured by an absence of symptoms.
All cases of chronic HEV in our treatment series developed in immunosuppressed patients, consistent with previous observations that chronic HEV in immunocompetent individuals is rare [5]. SOT recipients constituted the majority of chronic HEV cases observed in our series and all were treated with tacrolimus.
Kamar et al. identified that tacrolimus therapy is the main predictive factor for chronic HEV in SOT recipients. This may be related to a greater immunosuppressive effect compared with cyclosporine due to down regulation of T-cell responses against the virus [2]. Similarly, Choi et al. observed this association in renal transplant recipients who developed chronic HEV [6].
It has been postulated that a low platelet count is an independent predictive factor of chronic hepatitis in the HEV infection [7], although most patients in our series had a normal platelet count (mean 256 109L-1).
In contrast with other studies, none of our patients achieved SVR with reduction of immunosuppression alone and all were treated with ribavirin. Interestingly none of the patients cleared the virus spontaneously during a 3 month post diagnosis monitoring period, as recommended by the EASL 2018 clinical practice guidelines, which states to consider treatment after this time frame [8].
These practice guidelines had not yet been published when some of the earlier patients in our series were treated, thus there was heterogeneity in treatment stopping decisions. In 9 out of 11 ribavirin-naïve patients, cessation of treatment was guided by 2 negative serum HEV RNA PCR results 4 weeks apart, with parallel negative stool HEV RNA PCR results also available for 5 of these patients. None of these 9 patients relapsed. One patient, however, had a negative serum HEV RNA PCR and normal ALT 2 months after starting treatment but had persistent positive stool HEV RNA. Had the stool not been tested and the biochemistry/single negative serum HEV RNA PCR sample been used to guide treatment cessation, the patient may well have relapsed without the more prolonged course of treatment (6 months) which they eventually received.
Furthermore, the only patient who required ribavirin retreatment for HEV relapse had just a single serum HEV RNA used as a marker of viral clearance for their initial course (i.e. without stool test). The same patient had fluctuating stool HEV RNA positivity during re-treatment, resulting in a markedly prolonged (14 months), but successful re-treatment course. These results highlight the value of using 2 consecutive stool HEV RNA tests, 4 weeks apart, as a marker of SVR in immunosuppressed patients.
For the 10 patients who did not require re-treatment, the mean duration of the ribavirin course was 4.6 months (Figure 1).
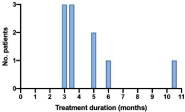
Figure 1: Duration of therapy for those who did not require re-treatment (n=10).
The EASL clinical practice guideline recommends a 3 month course of ribavirin monotherapy for chronic HEV and to cease treatment if the serum and stool are negative for RNA PCR at this time point, but to continue for a further 3 months if not. Our patients’ RNA testing was undertaken at a centralised UK reference laboratory distant from our centre, which may have artefactually increased the time between week 12 of treatment and the time of actual treatment completion (i.e. a processing and logistics delay). In addition, we elected to stop treatment only when 2 consecutive HEV RNA tests, 4 weeks apart, had been received for most of our patients. Despite these limitations, our series suggests that 3 months of ribavirin may be too short in some immunosuppressed patients. Consistent with this, a single HEV RNA PCR result prior to ceasing treatment (as per EASL 2018 guidance), was not a stringent enough predictor of SVR for all of our patients.
Despite the small numbers of patients treated in this series, ribavirin was highly effective at achieving SVR (91%), compared with SVR rates observed in other studies.8 This may be due in part to the individualised use of serial measurements of stool and serum RNA to determine optimal treatment duration.
In conclusion, this relatively small case series demonstrates that serial measurements of both serum and stool RNA can be used to optimise ribavirin treatment duration and outcome in immunosuppressed patients with chronic HEV infection. No cases of spontaneous clearance occurred within 3 months of diagnosis in our patients, despite dose reduction of immunosuppression, suggesting that HEV requires treatment in the majority of immunosuppressed patients. Validation of these findings in larger multi-centre studies will be useful, given the limited treatment options available for this condition.
References
- Kamar N, et al. Hepatitis E Virus and Chronic Hepatitis in Organ-Transplant Recipients. N Engl J Med. 2008; 358: 811-817.
- Kamar N, Rostaing L, Legrand-Abravanel F, Izopet J. How Should Hepatitis E Virus Infection Be Defined in Organ-Transplant Recipients? Am. J. Transplant. 2013; 13: 1935-1936.
- Kamar N, et al. Factors associated with chronic hepatitis in patients with hepatitis e virus infection who have received solid organ transplants. Gastroenterology. 2011; 140: 1481-1489.
- Dalton HR, et al. EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol. 2018; 68: 1256-1271.
- Wang Y, Liu S, Pan Q. Chronic hepatitis E in an immunocompetent patient. Clin Res Hepatol. Gastroenterol. pii: 2019; S2210-7401: 30184-30186.
- Choi M, et al. Prevalence and clinical correlates of chronic hepatitis E infection in German renal transplant recipients with elevated liver enzymes. Transplant. Direct. 2018; 4: 1-7.
- Kamar N, Izopet J, Dalton HR. Chronic Hepatitis E Virus Infection and Treatment. Journal of Clinical and Experimental Hepatology. 2013; 3: 134- 140.
- Kamar N, Izopet J, Pharm D, Tripon S, Bismuth M, Hillaire S, et al. Ribavirin for Chronic Hepatitis E Virus Infection in Transplant Recipients. N Engl J Med. 2014; 370: 1111-1120.