
Review Article
J Hepat Res. 2022; 7(1): 1048.
Distribution of Hepatitis C Virus Genotypes among Chronic HCV Patients in Greece from 2004 to 2019: Association of Genotypes and Viral Load with Gender and Age
Martinez-Gonzalez B1, Mpekoulis G2, Karatapanis S3, Lagou D2, Horefti E4, Papatheodoridis G5, Koskinas J6, Sgouras DN1, Mentis A4, Vassilaki N2* and Angelakis E4,7
1Laboratory of Medical Microbiology, Hellenic Pasteur Institute, Greece
2Laboratory of Molecular Virology, Hellenic Pasteur Institute, Greece
3First Department of Internal Medicine, General Hospital of Rhodes, Greece
4Diagnostic Department and Public Health Laboratories, Hellenic Pasteur Institute, Greece
5Department of Gastroenterology, Medical School of National and Kapodistrian University of Athens, General Hospital of Athens “Laiko”, Greece
6Second Department of Internal Medicine, “Hippokratio” General Hospital, Greece
7Aix Marseille Université, IRD, APHM, VITROME, IHUMéditerranée Infection, France
*Corresponding author: Vassilaki N, Laboratory of Molecular Virology, Hellenic Pasteur Institute, Athens, Greece
Received: October 11, 2022; Accepted: November 08, 2022; Published: November 15, 2022
Abstract
Identification of Hepatitis C Virus (HCV) genotypes and viral load are considered critical factors for the selection of the appropriate antiviral treatment regimens. In Greece, it is estimated that approximately 0.8% to 1.8% of the general population has been chronically infected with HCV. We aimed to evaluate the HCV genotype distribution trends and viral load levels in Greece from 2004 to 2019, according to gender and age, and to estimate the association of HCV genotypes with the viral load. A total of 6,824 serum samples from chronic HCV patients (4,614 males; average age of 47 ± 14.2), were assayed for HCV RNA and HCV genotyping. HCV genotype 3 was the most prevalent (42.4%) followed by genotype 1 (37.4%), genotype 4 (13.5%), genotype 2 (6.2%) and genotype 5 (0.3%). Genotype 6 was detected only in one Asian immigrant in 2012. The predominant subtypes were 3a (41.3%) and 1b (23.4%). HCV genotype distribution differed significantly (p < 0.001) between genders, with genotype 1 being more frequent in females, whereas genotype 3 was more common in males. The highest percentage of HCV infections was at the age-group 40-59 (44.8% of 6,097) and the lowest one at the age-group < 18 (0.6% of 6,097). Genotype 3 was highly represented in younger patients (19-39 age- group), while genotype 1 was more common in older ones (> 60 age-group). The distribution pattern of HCV genotypes during the 16-yearperiod study revealed a decreasing trend in the proportion of genotype 3 and a gradually increasing trend of genotype 1. HCV viral load was significantly lower (p < 0.001) in genotype 4 compared to genotypes 1, 2 and 3. Moreover, it was associated with gender, with elevated viral load in male individuals, and was positively correlated with age.
Keywords: Hepatitis C Virus; HCV Genotypes; Greece; Gender; Age; Viral load
Introduction
The Hepatitis C Virus (HCV) infection is a global health problem with an estimated prevalence rate of 1.1 % (range 0.9-1.4%) in the general population, corresponding to approximately 1.4% when considering those over 15 years of age [1]. The virus can cause both acute and chronic hepatitis. Acute HCV infection is usually asymptomatic or causes mild to moderate acute hepatitis, but it progresses to chronicity in the majority of cases. Chronic HCV infection can result in serious, even life-threatening health problems like cirrhosis, Hepatocellular Carcinoma (HCC), non-Hodgkin’s lymphomas, mixed cryoglobulinemia and liver-related death [2- 4]. Until recently, end-stage liver disease caused by chronic HCV infection is one of the most common reasons for liver transplantation in developed countries [5,6]. According to the World Health Organization (WHO), 58 million people have chronic HCV infection, with about 1.5 million infections occurring per year. In 2019, approximately 290,000 people died from HCV infection, mostly from cirrhosis and HCC [7]. The ultimate goal of the WHO is to reduce the global incidence of HCV infection to a negligible amount of less than 0.9 million chronic cases by the year 2030 [8].
Greece has made significant efforts towards HCV elimination over the recent years. The Hellenic National Plan for Hepatitis C is in alignment with the WHO goal to achieve eradication of HCV infection by 2030 [9]. Epidemiological studies in Greece demonstrate that the current prevalence of HCV chronically infected patients in the general population is estimated to range from 0.8% to 1.8% and between 1% to 1.9% in high-risk patient groups [9-11]. These data suggest that approximately 134,000 adults in the general population have chronic hepatitis C [9].
The HCV is an RNA virus, member of the Flaviviridae family, characterized by high genetic variability [12]. On the basis of phylogenetic analysis, there are 8 major genotypes (HCV 1-8), which comprise over 86 confirmed different subtypes of HCV [13-15]. HCV genotypes and subtypes information plays an important role in the pre-treatment evaluation of patients, as it is considered crucial for the selection of appropriate treatment regimens [13,16,17]. Despite HCV genotypes, HCV viral load assessment is an important tool particularly useful before initiating antiviral therapy, counting as a surrogate marker of antiviral response. The management of patients with HCV infection has changed substantially with the introduction of the highly effective oral Direct-Acting Antiviral Agents (DAAs), providing eradication rates higher than 95% [7], including populations that have been difficult to treat in the past [18,19]. Although pangenotypic DAAs have revolutionized HCV treatments, their high cost remains a major barrier to access in many countries [20]. So far, unless pangenotypic DAAs are made widely available, the initiation of a standard HCV treatment needs the determination of HCV genotype and subtype and the measurement of viral load level in order to improve treatment outcomes [13,16,17]. Moreover, HCV genotyping is essential for the evaluation of regional/national epidemiological status, as HCV genotypes are associated with the different transmission routes of HCV infection and have different geographical distribution [13]. However, the evidence of a correlation between HCV genotype and viral load remains controversial [21-23].
The aim of this study was to analyze the distribution pattern of HCV genotypes in chronically infected patients in a large sample size and over a sixteen-year period (2004-2019) according to gender and age, and to address the association with HCV viral load.
Materials and Methods
A retrospective study was carried out on HCV-positive patients who were successfully genotyped during the time period 2004-2019. Serum samples were collected from confirmed chronic HCV patients attending hospitals and clinical laboratories situated in different cities, from different geographical regions of Greece (Table 1). We tested HCV patients with long-term inflammation of the liver for at least six months, with positive HCV antibody and positive HCV RNA test. Written informed consent was requested from each patient at collection centers. Samples were sent to the Diagnostic Department at the Hellenic Pasteur Institute (HPI) at 2-8°C, if they were referred from hospitals located in the Capital City of Greece (Athens) and its metropolitan area or were shipped on dry ice when specimens were coming from other geographical regions of the country. Specimens were immediately stored at -80°C for further analysis at the Diagnostic Department of the HPI.
Variable
Number of cases
n = 6,824Percentage
%Gender
Male
4,614
67.6
Female
1,997
29.3
Unknown
213
3.1
Age (years)
<18
37
0.5
19-39
2,464
36.1
40-59
2,734
40.1
>60
862
12.6
Unknown
727
10.7
Year of genotyping
2004
255
3.7
2005
214
3.1
2006
275
4.0
2007
357
5.2
2008
301
4.4
2009
324
4.7
2010
247
3.6
2011
389
5.7
2012
530
7.8
2013
523
7.7
2014
529
7.8
2015
701
10.3
2016
774
11.3
2017
853
12.5
2018
294
4.3
2019
258
3.8
Residence (region/province)
Athens and Metropolitan area
4,210
61.7
Regions of Northern Greece
1,577
23.1
Regions of Western/Central Greece
331
4.9
Greek islands (Rhode, Crete, Chios)
706
10.3
Table 1: Characteristics of the patients’ population under study.
HCV RNA Load Determinations
Quantitation of HCV RNA was performed using the COBAS® AmpliPrep/COBAS® TaqMan HCV Quantitative Test, v.2.0 platform (Roche Molecular Systems, Branchburg, NJ), following the manufacturer’s specifications. During this long period, appropriate negative and positive control samples were included in each assay to ensure the comparability of the results.
HCV Genotype Identification
HCV RNA was extracted from patient’s serum using the QIAamp Viral RNA Mini kit (QIAGEN, GmbH, Germany) and cDNA was synthesized and amplified using the Versant® HCV Amplification 2.0 kit LiPA (Siemens, Healthcare Diagnostics). HCV genotyping was performed using the Versant® HCV Genotype 2.0 LiPA assay, a fully automated system (Siemens, Healthcare Diagnostics), designed for the use in reverse transcription and simultaneous amplification of the highly conserved 5’ untranslated region (5’UTR) and the core region of the HCV genome following the manufacture’s specifications.
Statistical Analysis
Statistical analysis of the data was performed with GraphPad Prism 9.0 package (GraphPad Software, Inc., San Diego, CA). Chisquared test was performed to find statistically significant differences in each genotype frequency among the populations of the different age groups and genders. Spearman’s correlation coefficient was implemented to assess the significance of the correlation between HCV viral load and age (continuous parameter). Mann-Whitney U test (non-parametric) was performed to analyze differences in viral load levels among genders for each genotype and subtype.
Regarding the age, comparison of HCV viral titters was performed with the non-parametric Kruskal-Wallis test. Multiple comparisons among age groups were carried out with the appropriate post-hoc test (Dunn’s test). Results with a p value < 0.05 were considered as statistically significant.
Ethical Statement
All study procedures were carried out in accordance with the declaration of Helsinki (18th World Medical Association Assembly), its subsequent amendments, the Greek regulations and guidelines, as well as the Good Clinical Practice guidelines (GCP) as defined by the International Conference of Harmonization. The study was also approved by the local ethics committees of participating hospitals.
Results
Descriptive Characteristics of the Patient Populations
A total of 6,824 serum samples were successfully genotyped and sub typed during the indicated study period 2004-2019. The characteristics of the studied population including gender, age, year of genotyping and residence are depicted in (Table 1). Of the total HCV infected patients, 4,614 were males with a Male to Female (M/F) ratio of 2.3, whereas their mean age was 47 ± 14.2 years (range 2-85 years). The average annual number of HCV cases analyzed herein, was 340.5 (range 214-853). The highest percentage of HCV cases was found in Athens and Metropolitan area (61.7%, 4,210/6,824), a region with a population of 3.8 million, representing one third of the total population of Greece. HCV infected patients were categorized into 4 age groups: minors (< 18 years of age), younger (19-39 age group), middle age (40-59 age group) and senior patients (> 60 years of age). The majority of HCV infected patients belonged to the younger and the middle age groups (36.1% and 40.1%, respectively).
HCV Genotype Distribution
The distribution of HCV genotypes/subtypes in the study population is depicted in (Figure 1). Genotype 3 was the most prevalent (n=2,893, 42.4%), followed by genotype 1 (n=2,551, 37.4%), genotype 4 (n=922, 13.5%), genotype 2 (n=421, 6.2%) and genotype 5 (n=18, 0.3%). One case of genotype 6 (0.01%) was detected in 2012 from an Asian immigrant. Regarding the HCV subtypes, genotype 1b (n=1,595, 23.4%) was more frequently observed than genotype 1a (n=956, 14%). Genotype 3a was found in 41.3% (n=2,821) of the cases, while 72 patients were infected with genotypes 3b, 3c and 3k. Among the examined individuals, 18 patients were classified with mixed genotype infections corresponding to 0.3% of the total sample population. Mixed infections consisted of two different HCV genotypes; genotypes 1 and 3 (n=9, 50%), genotypes 1 and 2 (n=3, 16.7%), genotypes 2 and 3 (n=3, 16.7%), genotypes 3 and 4 (n=2, 11.1%) and genotypes 2 and 4 (n=1, 5.5%).
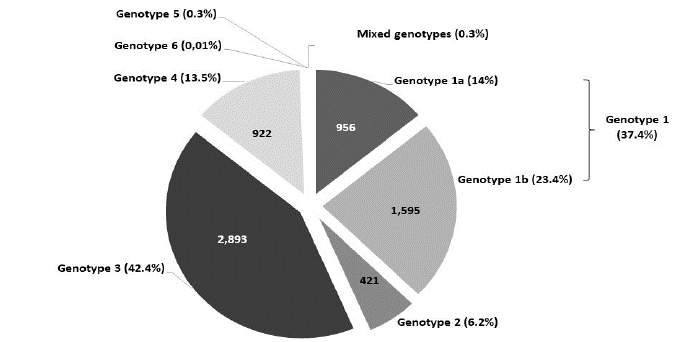
Figure 1: Relative distribution of HCV genotypes and their subtypes among 6,824 patients during a 16-year period (2004-2019) in Greek population.
Determination of the distribution frequency of HCV genotypes according to the year of collection showed a predominance of genotypes 1 and 3, with significant fluctuation throughout the study period (Figure 2). Regarding genotype 1, a considerable increase was observed from 32.2% in 2004 to 43.5% in 2014 (p < 0.0024), followed by a significant decrease from 40.2% in 2015 to 28.9% in 2018 (p < 0.0025). This was accompanied by a parallel decrease in genotype 3 from 47.8% to 36.7%, in period 2004-2014 (p < 0.0031), followed by an increase from 34.4% to 43.8%, in period 2015-2018 (p < 0.0083). Genotypes 2 and 4, which represented the fourth and the third largest groups, respectively, seemed to be equally distributed without significant variations during the 16-year study period (Figure 2).
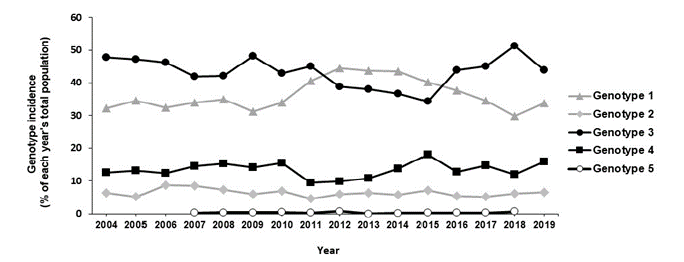
Figure 2: HCV genotypes distribution pattern from 2004 to 2019 in Greece. The total population of patients for each year was set to 100%. Data presented as
XY dot plot with fitted connecting line. Each dot represents the percentage of each genotype incidence in the specific year.. The total population of patients for each year was set to 100%. Data presented as
XY dot plot with fitted connecting line. Each dot represents the percentage of each genotype incidence in the specific year.
Frequency of HCV Genotypes According to Gender and Age
We next aimed to elucidate the association of the gender with the genotype frequency in 6,611 HCV-RNA positive samples, corresponding to a sub-population of patients whose gender was reported. The distribution of HCV genotypes in both genders is depicted in (Table 2 and Figure 3). The predominant genotype among male individuals was genotype 3 (48.3%), followed by genotype 1 (32.2%), genotype 4 (13.4%) and genotype 2 (5.6%) (Figure 3). However, among females, the most frequent genotype was genotype 1 (48.2%) followed by genotype 3 (30%), genotype 4 (13.6%) and genotype 2 (7.5%). Statistically significant differences were observed in the frequency of genotypes 1, 2, 3 and 5 between men and women. Genotypes 1 and 5 were more frequent in women (χ²=152.12; p < 0.0001, χ²=4.361; p = 0.0368, respectively), while genotypes 2 and 3 were more common in men (χ²=8.33; p = 0.0039, χ²=190.67; p < 0.0001, respectively) (Table 2). No significant differences in the frequency of the genders were observed for genotype 4 and mixed genotypes (χ²=0.05; p = 0.8164 and χ²=0.99; p = 0.3193, respectively) (Table 2).
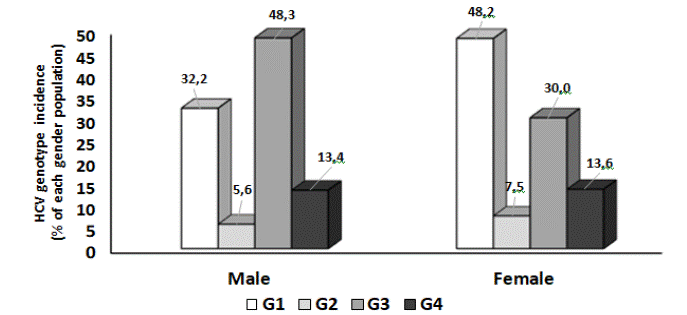
Figure 3: Association of gender with HCV genotype frequency. Data are presented as bar graphs. Same-colored bars refer to each genotype incidence in
both genders.
HCV genotype
Gender
Male
Female
p value*
n
%
n
%
Genotype 1
1,485
32.2
962
48.2
<0.0001
subtype 1a
757
16.4
236
11.8
<0.0001
subtype 1b
728
15.8
726
36.6
<0.0001
Genotype 2
259
5.6
150
7.5
0.0039
Genotype 3
2,230
48.3
599
30.0
<0.0001
subtype 3a
2,184
47.3
582
29.1
<0.0001
other subtypes 3
46
1.0
17
0.9
0.6730
Genotype 4
617
13.4
272
13.6
0.8164
Genotype 5
8
0.2
10
0.5
0.0368
Genotype 6
-
-
1
0.05
-
Mixed genotypes
15
0.3
3
0.2
0.3193
Total
4,614
69.8
1,997
30.2
*p values were calculated by chi-squared analysis to compare the frequency of each genotype among gender populations, taking into account the number of the infected patients with a specific genotype and the infected ones with all other genotypes.
Table 2: HCV genotype frequencies (%) according to gender in 6,611 chronic HCV patients. The total population of each gender was set to 100%.
In addition to gender, we examined whether age is associated with the genotype incidence in 6,097 patient samples. Age data reported in our study are contained in (Table 3). HCV genotypes distribution varied significantly among age groups as presented in (Figure 4). Genotype 1 was dominant in the senior group (> 60 years), while genotype 3 prevailed the other patient groups (< 18, 19-39 and 40-59 years). Genotype 2 was more frequently observed in the minor age group, while genotype 4 was more common in older patients (age > 40 years). Most of the patients infected with genotype 5 were in the senior age group.
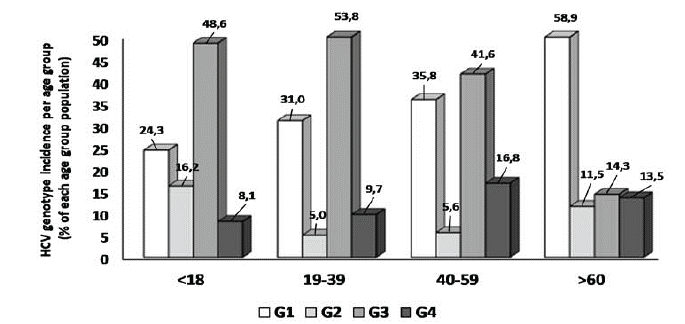
Figure 4: HCV genotypes dominance among age groups. Data are presented as bar graphs. Same- colored bars refer to each genotype incidence in each age
group population.. Data are presented as bar graphs. Same- colored bars refer to each genotype incidence in each age
group population.
Years of age n (%)
HCV genotype
< 18
19-39
40-59
> 60
unknown
p value*
Genotype 1
9
(24.3)
763
(31.0)
980
(35.8)
514
(58.9)
285
(39.7)
<0.0001
subtype 1a
2
(5.4)
387
(15.7)
352
(12.9)
68
(7.8)
129
(18.0)
<0.0001
subtype 1b
7
(18.9)
344
(14.0)
584
(21.4)
435
(49.9)
149
(20.8)
<0.0001
other subtypes 1
-
32
(1.3)
44
(1.6)
11
(1.3)
7
(1.0)
-
Genotype 2
6
(16.2)
123
(5.0)
152
(5.6)
100
(11.5)
40
(5.6)
<0.0001
Genotype 3
18
(48.6)
1,325
(53.8)
1,136
(41.6)
125
(14.3)
289
(40.3)
<0.0001
subtype 3a
17
(45.9)
1,293
(52.5)
1,105
(40.4)
123
(14.1)
283
(39.5)
<0.0001
other subtypes 3
1
(2.7)
32
(1.3)
31
(1.1)
2
(0.2)
6
(0.8)
-
Genotype 4
3
(8.1)
240
(9.7)
459
(16.8)
118
(13.5)
102
(14.2)
<0.0001
Genotype 5
-
1
(0.04)
2
(0.1)
15
(1.7)
-
-
Genotype 6
-
1
(0.04)
-
-
-
-
Mixed genotypes
1
(2.7)
11
(0.4)
5
(0.2)
-
1
(0.1)
-
Total (n=6,824)
37
(0.5)
2,464
(36.1)
2,734
(40.1)
872
(12.8)
717
(10.5)
*p values were calculated by chi-squared analysis to compare the populations of each genotype within the different age groups.
Table 3: HCV genotype frequencies according to age groups in 6,097 chronic HCV patients. The total population of each age group was set to 100%.
Variation of HCV Viral Load among Different Genotypes
Viral load quantification was carried out in 5,737 HCV positive serum samples. Univariate analysis was performed to analyze the relation between viral load and HCV genotypes. No significant differences in the viral load levels were found among genotypes 1, 2 and 3 (p = 0.6048) (Figure 5). In contrast, when viral titers for genotype 4 were compared to the ones for genotypes 1, 2 and 3, there was a significant difference (p < 0.0001). The medians of viral load in patients infected with genotypes 1, 2, 3 or 4 were 7.7 x 105 IU/ml, 8.3 x 105 IU/ml, 7.1 x 105 IU/ml and 5.4 x 105 IU/mL, respectively. Regarding genotype 5, viral load levels for only 14 samples were available (median= 1.3 x 106 IU/mL). Therefore, this genotype was not included in the viral load comparison.
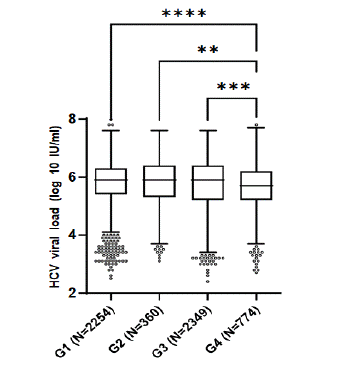
Figure 5: Comparison of HCV viral load among genotypes. Data are
presented as box plots. The central line corresponds to the median value
of its group; box edges, the 25th to 75th centiles and whiskers represent the
range of values excluding outliners. p value was calculated using the nonpara-
metric Kruskal-Wallis test. **p = 0.0017, ***p = 0.0001, ****p < 0.0001.
As HCV genotypes 1 and 3 accounted for 80.2% of HCV infections, we compared viral load distribution in the 16-year period between these two dominant groups of genotypes. Viral load distribution was classified into 8 categories based on each viral load levels (Figure 6, Table 4). According to our data, we observed that the frequency of patients having a viral load higher than 3 x 106 IU/ ml was greater among patients infected with genotype 3 compared to genotype 1 (Figure 6).
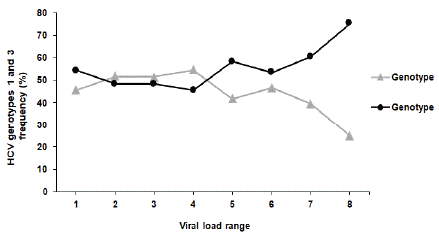
Figure 6: HCV genotype dominance among viral load ranges. Data
presented as XY dot plot with fitted connecting line. Each dot represents the
percentage of each genotype incidence in the specific viral load category.
Viral load (v) zones
Genotype 1 (%)
Genotype 3 (%)
n
1
v < 5x105
45.6
54.4
2,229
2
5x105 < v < 1x106
51.7
48.3
838
3
1x106 < v < 2x106
51.6
48.4
821
4
2x106< v < 3x106
54.6
45.5
429
5
3x106< v < 4x106
41.7
58.3
283
6
4x106< v < 5x106
46.6
53.4
189
7
5x106< v < 1 x107
39.5
60.5
423
8
v > 1x107
24.8
75.2
206
Table 4: HCV genotypes 1 and 3 frequency (%) within 8 different viral load ranges. The total population of infected patients with both genotypes was set to 100% for each viral load range.
Association of HCV Viral Load Levels with Gender and Age
A relation between viral load and patient’s gender was detected. In 5,580 samples, a sub-population of the total samples with reported HCV viral load and gender, approximately 69.1% (3,856 patients) were male and they had significantly higher viral loads than females (median values male 8.1 x 105 IU/ml versus female 5.3 x 105 IU/ml, p < 0.0001). More precisely, we observed enhanced levels of HCV viral load in male individuals compared to female ones for genotypes 1 (p = 0.0002), 3 (p < 0.0001) and 4 (p = 0.0448) (Figure 7).
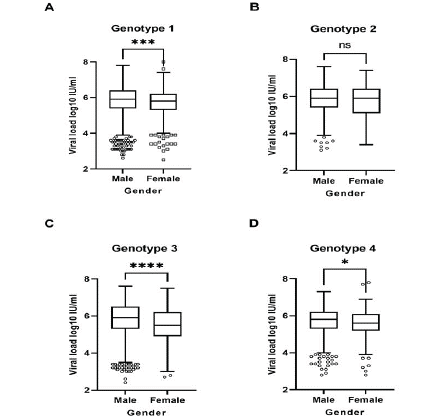
Figure 7: Comparison of HCV viral load levels among gender per genotype. Data are represented as box plots. The central line corresponds for the median
value of each group; box edges, the 25th to 75th centiles and whiskers represent the range of values excluding outliners. p values were calculated using the nonparametric
Man Whitney U test. * p = 0.0448, ***p = 0.0002, ****p < 0.0001.
Then, we aimed to elucidate the possibility of patients’ age to be related with HCV viral titer. For this purpose, we analyzed 5,109 patients’ samples, whose age and viral load levels were reported. For all genotypes we observed a relation between age and HCV viral load as shown in (Figure 8). More specifically, for genotypes 1, 3 and 4 the viral load levels were elevated in the middle age and senior patient groups (40-59, > 60 years), whereas for genotype 2 there was no statistically significant alteration among the age groups (Figure 8). Moreover, when examining the subtypes of genotype 1, 1a and 1b, separately, we observed that the difference in the viral load of the senior (> 60 years) compared to the younger (19-39) patients was more intense for subtype 1b versus 1a (Supplementary data). In consistence, when age was analyzed as a continuous parameter, a positive correlation between HCV viral load and age was detected for genotypes 1 (r=0.2252, p < 0.0001), 3 (r=0.1883, p < 0.0001) and 4 (r=0.1937, p < 0.0001) (Figure 9). On the contrary, there was no significant correlation between viral load and age for genotype 2 (r=0.0114, p = 0.8383).
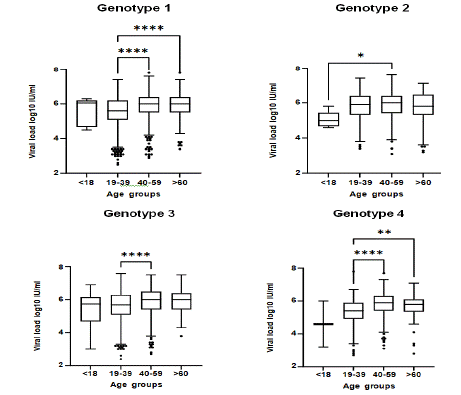
Figure 8: Comparison of HCV viral load levels among the different age groups per genotype. Data are represented as box plots. The central line corresponds
for the median value of each group; box edges, the 25th to 75th centiles and whiskers represent the range of values excluding outliners. p values were calculated
using the non-parametric Kruskal Wallis test. Multiple comparisons were performed with Dunn’s post-hoc test. *p = 0.0443, **p = 0.0058 and ****p < 0.0001.
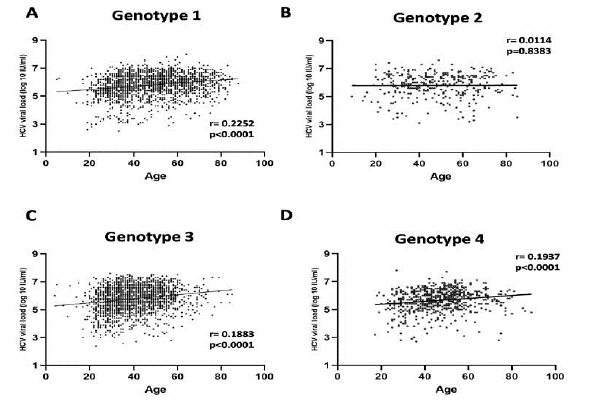
Figure 9: Correlation of HCV viral load levels with age per genotype. Data are represented as XY scatter plot and fitted linear regression lines. Spearman’s
correlation coefficient (r) and p values (p) were calculated.
Discussion
The introduction of highly effective and well tolerated pangenotypic DAAs has greatly increased HCV treatment options [24]. However, pangenotypic regimens may not be used in several countries due to their high-cost and country-specific negotiations. Thus, HCV genotyping remains an essential tool for the control and therapeutic management of HCV infection, while it is mandatory for the evaluation of regional/national epidemiological status. In this context, we monitored the distribution patterns of circulating HCV genotypes and the viral load levels, and associated them with gender and age, for a 16-year period in Greece.
The HCV genotype that infects a patient depends on the route of transmission of the virus [9,18]. Until 1980s the main route of HCV transmission was via blood transfusions, long-term hemodialysis, and medical surgeries as a result of poor infection control practices, resulting in the dominance of genotype 1 and more precisely of subtype 1b. Since 1990, the significant increase of intravenous drugusers population, combined with the increased blood transfusion safety and the improvement of healthcare conditions in Europe, including Greece (1992 was the year of compulsory anti-HCV blood and blood products screening in our country), led to the increase of genotype 3 prevalence [9,10,18,25-28]. The predominance of genotype 3 has also been favored by the low effectiveness of its treatment with first generation DAAs protease inhibitors [29], as well as the migration towards Europe of people from countries where genotype 3 is endemic, such as India and Pakistan [30,31]. In consistence with the above, our data showed that in Greece, for the period 2004-2019 in a high number of samples (6,824), the two dominant HCV genotypes were 1 and 3, followed by genotypes 2 and 4, with percentages of 37.4%, 42.4%, 6.2% and 13.5% of total cases, respectively. Genotype 3 dominated during the 12 of the 16-yearsstudy (periods 2004-2011 and 2016-2019), while genotype 1 was more frequently observed from 2012-2015. The increase of genotype 3 prevalence and the simultaneous reduction of genotype 1 was also observed in the study of Gioula et al. [27], which was performed in a smaller number of HCV positive patients mainly from Northern Greece. Our data are quite different from other reported European studies where genotype 1 is the predominant genotype followed by genotype 3 [32]. The incidence of genotype 5 in our study population was low (0.3%) corroborating that this genotype is of a rare occurrence in Greece and is mainly reported on the island of Rhodes, in South- Eastern Greece [33].
Among genders, we found a higher proportion of males, with an M/F ratio of 2.3. This finding agrees with already published studies from European and South Asian countries [31,34,35]. In the reported study from Sindhi (Pakistan) [31], 1,471 patients’ samples from six cities in Sindhi were tested, and significant gender-dependent differences in infection frequency were found, by ~5-fold higher levels in male individuals as compared to female ones. Furthermore, based on our findings, significant alterations were observed between men and women in the frequency of genotypes 1, 2 and 3. The dominant HCV genotype in men was genotype 3, followed by genotype 1 (48.3%, 32.2% of total male individuals, respectively). Accordingly, in a study from Hazara (Pakistan), dominance of genotype 3 was noticed in men, followed by genotype 1 and 2 [36]. This was also observed in other studies from Greece [18,26]. On the other hand, in our cohort, genotype 1 was the predominant one in women, followed by genotype 3 (47.3%, 30% of total female individuals, respectively). The same observations were obtained by other also studies in Greece, concluding of genotype 1 dominance in women [10,18,26,27].
Concerning the association of HCV genotypes with the age, we noted that genotype 3 was the most frequent one in minor (< 18 years), younger (19-39) and middle age (40-59) groups, which agrees with already published studies around the world [26,31,37]. These results can be attributed to extended intravenous drug use, as well as to the higher tendency for tattooing and piercing, in these age groups [38]. On the contrary, in our cohort, genotype 1, mainly subtype 1b, dominated in the oldest age group (> 60 years). This is expected due to higher frequency of blood transfusions and medical surgeries in this age range [37,38], or could be a long-term effect of the past HCV outbreak in Central and Southern Europe, which was transfusionrelated [39]. The dominance of genotype 1, and more precisely of the subtype 1b in older people, has been also supported by other studies for Greek patients [18,26].
The observed HCV genotype prevalence fluctuations through the years under study (2004-2019), and particularly for genotypes 1 and 3, could be attributed to the availability and accessibility to the recommended treatments at each time period. More specifically, during the years 2004-2010 and concerning a large part of the Greek population presented in our study (n=1,973), combination regimens of pegylated interferon-α (Peg-INF-α) with Ribavirin (RBV) were more successful in patients with HCV genotypes 2 and 3 infections, leading to higher Sustained Virological Response (SVR) rates (64.8% and 72.9%, respectively), as compared to patients with genotypes 1 and 4 infections (37.6% and 37.1%, respectively) [40,41]. Between 2011 and 2015, triple therapies consisting of one first-generation protease inhibitor (boceprevir or telaprevir) + Peg-INF-α/RBV were available in the treatment of subjects with genotype 1. Unfortunately, these regimens were moderately active for genotype 2 and appeared to have very limited activity against genotype 3 [42]. The achieved SVR12, in a cohort of HCV genotype 1-infected and treatment-naïve patients, including individuals participating in the present study, was 56.6% and 62.9% for boceprevir in combination with Peg-INF-α-2a/ RBV and Peg-INF-α-2b/RBV, respectively, and 65.3% and 58.6% for telaprevir in combination with Peg-INF-α-2a/RBV and Peg-INF- α-2b/RBV, respectively [43]. The studied samples we collected after 2015 (n=2,880) concern patients from a cohort of HERACLIS Hellenic Multicenter real-life clinical study, that received combination of two DAAs (HCV polymerase inhibitor sofosbuvir and second generation protease or HCV NS5A in- hibitors) ± RBV. These combinations showed significantly increased SVR and treatment completion with estimated overall rates > 90%, with some heterogeneity within genotypes [9,44,45]. More specifically for genotype 3, the combined DAAs treatment using sofosbuvir/velpatasvir ± RBV achieved SVR12 in 94% of patients who completed treatment and 12 weeks posttreatment follow-up.
Until today, it is arguable whether certain HCV genotypes affect HCV RNA levels. Specifically, studies have shown that patients infected with genotype 1 had higher tendency to have greater viral load than those infected with genotypes 2 and 3 [21]. Others though failed to find any significant association [23]. Our data showed significant differences in HCV RNA levels, only when comparing genotypes 1, 2 and 3 with genotype 4. Additionally, when we grouped viral loads in 8 major ranges for the two most frequently observed genotypes, 1 and 3, we observed that people infected with genotype 3 had higher tendency to have viral titers greater than 3 x 106 IU/ ml, as compared to individuals infected with genotype 1. However, when comparing the viral load levels of the total sample population between these two genotypes, there was no significant difference. This is possibly due to the limited number of samples in the higher viral load ranges.
Concerning the gender, we detected elevated levels of HCV RNA in male individuals in genotypes 1, 3 and 4. This result is in accordance with an already published study, where higher levels of HCV viral load was observed in male individuals with age > 40 years [46]. Additionally, we observed differences in the amounts of HCV RNA concerning the age. Specifically, we detected higher HCV viral titers in the older age groups (40-59, > 60) compared to the younger ones (< 18, 19-39), which was also reflected in the positive correlation of viral load with age for genotypes 1, 3 and 4 and could be attributed to the extent of liver disease. The aforementioned findings regarding the association of viral load with age agree with already published studies around the world [47,48].
The advantage of this study over the previous ones conducted in Greek patients is the significantly higher number of samples analyzed, as well as the more extended sample collection period. On the other hand, missing data regarding age, gender and viral load for a number of patients was a limitation.
Conclusions
Herein, we report a large-scale study of HCV genotypes distribution and viral load levels in Greece during a 16-year collection period, and their association with gender and age. In conclusion, we provided evidence for the predominance of genotype 3 during the vast majority of the years under study, followed by genotype 1. Moreover, our data highlighted an association of HCV genotype distribution with gender and age. Genotype 1 was more frequent in females and in patients with age > 60 years, whereas genotype 3 was more common in males and in younger age groups. These data are possibly related to the transmission route of the virus. Differences in viral titers were clearly observed only for genotype 4, as compared to other genotypes. Notably, viral load levels were also associated with gender, being elevated in male individuals, and were positively correlated with age.
Finally, physicians should consider the results of the current and other similar studies on the surveillance of circulating HCV genotypes, as this is crucial for epidemiological analysis and for the guidance of therapeutic decisions especially in those countries in which the pangenotypic DAAs are not yet available or widely used.
Acknowledgments
The authors would like to thank Mr. Antonios Kalliaropoulos, Technical Research Analyst at the Diagnostic Department and Public Health Laboratories, Hellenic Pasteur Institute, for his valuable experimental and scientific contribution to this study; they would also like to thank Dr. I. Ketikoglou (Hippokration General Hospital of Athens, Athens, Greece), Dr. D. Dimitroulopoulos (Agios Savvas Hospital, Athens, Greece), Dr. K. Mimidis (University Hospital of Alexandroupolis, W. Thrace, Greece), Dr. I. Eleusiniotis (Agioi Anargyroi General and Oncology Hospital, Athens, Greece), Dr. I. Goulis (Aristotelian University of Thessaloniki, Thessaloniki, Greece), Dr. A. Protopapas (AHEPA Hospital, Thessaloniki, Greece), Dr. M. Tsagkaris (National Health Dept, Sismanoglion Gen- eral Hospital of Athens, Greece), Dr. S. Manolakopoulos (Hippokration General Hospital of Athens, Athens, Greece), Dr. I. Xinotroulas (General Hospital of Lamia, Greece), Dr. E. Manesis (Athens University Medical School, Athens, Greece), Dr. A Gatopoulou (University Hospital of Alexandroupolis, W. Thrace, Greece), Dr. S Papantoniou (General Hospital of Kavala, Greece), Dr. M Deutsch (Hippokration General Hospital of Athens, Athens, Greece), Dr. P. Ioannidou (Athens University Medical School, Athens, Greece), Dr. I. Vlachoiannakos (Athens University Medical School, Laiko General Hospital, Athens, Greece), Dr. I Vafeiadis (Athens University Medical School, Laiko General Hospital, Athens, Greece), Dr. N. Papadopoulos (417 Army Share Fund Hospital of Athens, Greece), Dr. D. Glaros (University Hospital of Alexandroupolis, W. Thrace, Greece), Dr. A. Alexopoulou (Hippokration General Hospital of Athens, Athens, Greece), Dr. M. Mela (“Evangelismos-Ophthalmiatreion Athinon – Polykliniki” General Hospital of Athens, Greece), Dr. C. Drakoulis (General Hospital of Nikea, Piraeus, Greece), Dr. C. Triantos (University Hospital of Patras, Patras, Greece), Dr. E. Anagnostopoulou (General Hospital of Chania St. George, Crete, Greece), Dr. K. Dampos (General Hospital of Ioan- nina G. Hatzikosta, Ioannina, Greece), Dr. C. Palamarou (Athens Medical Center, Greece) and Dr. N. Anastasiadis (IKA Hospital Thessaloniki, Thessaloniki, Greece) for their cooperation in the study.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding
The Diagnostic Department of the Hellenic Pasteur Institute received research funding from private sources (Janssen-Cilag Pharmaceuticals, Merck Sharp & Dohme S.A. and AbbVie Pharmaceuticals S.A.). These grants were supervised by A.M. and were limited to basic epidemiology and molecular diagnostic research. G.M. was supported by an excellence PhD scholarship from Hellenic Pasteur Institute in the context of NOSTOS donation (principal investigator N.V.).
References
- Stasi C, Silvestri C, Voller F. Update on Hepatitis C Epidemiology: Unaware and Untreated Infected Population Could Be the Key to Elimination. SN comprehensive clinical medicine. 2020; 2: 2808-15.
- Lauer GM, Walker BD. Hepatitis C virus infection. The New England journal of medicine. 2001; 345: 41-52.
- Axley P, Ahmed Z, Ravi S, Singal AK. Hepatitis C Virus and Hepatocellular Carcinoma: A Narrative Review. Journal of clinical and translational hepatology. 2018; 6: 79-84.
- Khaled H, Abu-Taleb F, Haggag R. Hepatitis C virus and non-Hodgkin’s lymphomas: A minireview. Journal of advanced research. 2017; 8: 131-7.
- Wang S, Toy M, Hang Pham TT. Causes and trends in liver disease and hepatocellular carcinoma among men and women who received liver transplants in the U.S., 2010-2019. 2020; 15: e0239393.
- Müller PC, Kabacam G, Vibert E, Germani G, Petrowsky H. Current status of liver transplantation in Europe. International journal of surgery (London, England). 2020; 82s: 22-9.
- World Health Organization. Hepatitis [cited 2022 17 May 2022]. Available from: https://www.who.int/health-topics/hepatitis#tab=tab_3.
- World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis. Geneva: World Health Organization, 2016 2016. Report No.: Contract No.: WHO/HIV/2016.06.
- Papatheodoridis GV, Goulis J, Sypsa V, Lionis C, Manolakopoulos S, Elefsiniotis I, et al. Aiming towards hepatitis C virus elimination in Greece. Annals of gastroenterology. 2019; 32: 321-9.
- Triantos C, Konstantakis C, Tselekouni P, Kalafateli M, Aggeletopoulou I, Manolakopoulos S. Epidemiology of hepatitis C in Greece. World journal of gastroenterology. 2016; 22: 8094-102.
- Papatheodoridis G, Sypsa V, Kantzanou M, Nikolakopoulos I, Hatzakis A. Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey. Journal of viral hepatitis. 2015; 22: 409-15.
- Echeverría N, Moratorio G, Cristina J, Moreno P. Hepatitis C virus genetic variability and evolution. World journal of hepatology. 2015; 7: 831-45.
- Hedskog C, Parhy B, Chang S, Zeuzem S, Moreno C, Shafran SD, et al. Identification of 19 Novel Hepatitis C Virus Subtypes-Further Expanding HCV Classification. Open forum infectious diseases. 2019; 6: ofz076.
- Borgia SM, Hedskog C, Parhy B, Hyland RH, Stamm LM, Brainard DM, et al. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. The Journal of infectious diseases. 2018; 218: 1722-9.
- Table 1 - Confirmed HCV genotypes/subtypes (March 2022) International Committee on Taxonomy of Viruses (ICTV) [cited 2022 17 may 2022]. Available from: https://talk.ictvonline.org/ictv_wikis/flaviviridae/w/sg_ flavi/634/table-1---confirmed-hcv-genotypes-subtypes-march-2022.
- Sherman KE. Therapeutic approach to the treatment-naive patient with hepatitis C virus genotype 1 infection: a step-by-step approach. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012; 55: 1236-41.
- Chronic Hepatitis C Virus Infection: Developing Direct-Acting Antiviral Drugs for Treatment Guidance for Industry, Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER), November 2017 Clinical/Antimicrobial (2017).
- Katsoulidou A, Sypsa V, Tassopoulos NC, Boletis J, Karafoulidou A, Ketikoglou I, et al. Molecular epidemiology of hepatitis C virus (HCV) in Greece: temporal trends in HCV genotype-specific incidence and molecular characterization of genotype 4 isolates. Journal of viral hepatitis. 2006; 13: 19-27.
- Nakano T, Lau GM, Lau GM, Sugiyama M, Mizokami M. An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver international: official journal of the International Association for the Study of the Liver. 2012; 32: 339-45.
- Mohamed AA, Elbedewy TA, El-Serafy M, El-Toukhy N, Ahmed W, Ali El Din Z. Hepatitis C virus: A global view. World journal of hepatology. 2015; 7: 2676-80.
- Rong X, Lu L, Wang J, Xiong H, Huang J, Chen J, et al. Correlation of viral loads with HCV genotypes: higher levels of virus were revealed among blood donors infected with 6a strains. PloS one. 2012; 7: e52467.
- Navaneethan U, Kemmer N, Neff GW. Predicting the probable outcome of treatment in HCV patients. Therapeutic advances in gastroenterology. 2009; 2: 287-302.
- Afridi SQ, Ali MM, Awan F, Zahid MN, Afridi IQ, Afridi SQ, et al. Molecular epidemiology and viral load of HCV in different regions of Punjab, Pakistan. Virology journal. 2014; 11: 24.
- Zoratti MJ, Siddiqua A, Morassut RE, Zeraatkar D, Chou R, van Holten J, et al. Pangenotypic direct acting antivirals for the treatment of chronic hepatitis C virus infection: A systematic literature review and meta-analysis. E Clinical Medicine. 2020; 18: 100237.
- Rigopoulou EI, Stefanidis I, Liaskos C, Zervou EK, Rizos C, Mina P, et al. HCV-RNA qualitative assay based on transcription mediated amplification improves the detection of hepatitis C virus infection in patients on hemodialysis: results from five hemodialysis units in central Greece. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2005; 34: 81-5.
- Raptopoulou M, Touloumi G, Tzourmakliotis D, Nikolopoulou G, Dimopoulou M, Giannoulis G, et al. Significant epidemiological changes in chronic hepatitis C infection: results of the nationwide HEPNET-GREECE cohort study. Hippokratia. 2011; 15: 26-31.
- Gioula G, Sinakos E, Gigi E, Goulis I, Vasiliadis T, Minti F, et al. ‘Distribution of Hepatitis C Virus genotypes in northern Greece in the last decade: descriptive analysis and clinical correlations’. Global health, epidemiology and genomics. 2019; 4: e5.
- Kranidioti H, Chatzievagelinou C, Protopapas A, Papatheodoridi M, Zisimopoulos K, Evangelidou E, et al. Clinical and epidemiological characteristics of hepatitis C virus-infected people who inject drugs: a Greek descriptive analysis. Annals of gastroenterology. 2018; 31: 598-603.
- de Leuw P, Stephan C. Protease inhibitors for the treatment of hepatitis C virus infection. GMS infectious diseases. 2017; 5: Doc08.
- Uddin G, Shoeb D, Solaiman S, Marley R, Gore C, Ramsay M, et al. Prevalence of chronic viral hepatitis in people of south Asian ethnicity living in England: the prevalence cannot necessarily be predicted from the prevalence in the country of origin. Journal of viral hepatitis. 2010; 17: 327-35.
- Riaz S, Bashir MF, Haider S, Rahid N. Association of genotypes with viral load and biochemical markers in HCV-infected Sindhi patients. Brazilian journal of microbiology: publication of the Brazilian Society for Microbiology. 2016; 47: 980-6.
- Petruzziello A, Loquercio G, Sabatino R, Balaban DV, Ullah Khan N, Piccirillo M, et al. Prevalence of Hepatitis C virus genotypes in nine selected European countries: A systematic review. Journal of clinical laboratory analysis. 2019; 33: e22876.
- Karatapanis S, Tsoplou P, Papastergiou V, Vasiageorgi A, Stampori M, Saitis I, et al. Hepatitis C virus genotyping in Greece: unexpected high prevalence of genotype 5a in a Greek island. Journal of medical virology. 2012; 84: 223- 8.
- Aguilera A, Navarro D, Rodriguez-Frias F, Viciana I, Martinez-Sapina AM, Rodriguez MJ, et al. Prevalence and distribution of hepatitis C virus genotypes in Spain during the 2000-2015 period (the GEHEP 005 study). Journal of viral hepatitis. 2017; 24: 725-32.
- Zago D, Pozzetto I, Pacenti M, Brancaccio G, Ragolia S, Basso M, et al. Circulating Genotypes of Hepatitis C Virus in Italian Patients before and after the Application of Wider Access Criteria to HCV Treatment. Open Microbiology Journal. 2022; 16.
- Ali A, Nisar M, Ahmad H, Saif N, Idrees M, Bajwa MA. Determination of HCV genotypes and viral loads in chronic HCV infected patients of Hazara Pakistan. Virology journal. 2011; 8: 466.
- Savvas SP, Koskinas J, Sinani C, Hadziyannis A, Spanou F, Hadziyannis SJ. Changes in epidemiological patterns of HCV infection and their impact on liver disease over the last 20 years in Greece. Journal of viral hepatitis. 2005; 12: 551-7.
- Ampuero J, Romero-Gomez M, Reddy KR. Review article: HCV genotype 3 - the new treatment challenge. Alimentary pharmacology & therapeutics. 2014; 39: 686-98.
- Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. Journal of hepatology. 2008; 48: 148-62.
- Deutsch M, Hadziyannis SJ. Old and emerging therapies in chronic hepatitis C: an update. Journal of viral hepatitis. 2008; 15: 2-11.
- Anagnostou O, Manolakopoulos S, Bakoyannis G, Papatheodoridis G, Zisouli A, Raptopoulou-Gigi M, et al. Genotype 4 HCV infection is difficult to cure with pegylated interferon and ribavirin. Results from a Greek Nationwide Cohort Study. Hippokratia. 2014; 18: 57-64.
- Wilby KJ, Partovi N, Ford JA, Greanya E, Yoshida EM. Review of boceprevir and telaprevir for the treatment of chronic hepatitis C. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2012; 26: 205-10.
- Mangia A, Foster GR, Berg CP, Curescu M, Ledinghen V, Habersetzer F, et al. Efficacy and safety profile of boceprevir- or telaprevir-based triple therapy or dual peginterferon alfa-2a or alfa-2b plus ribavirin therapy in chronic hepatitis C: the real-world PegBase observational study. Annals of gastroenterology. 2017; 30: 327-43.
- Papatheodoridis GV, Koskinas J, Goulis Ι, Sinakos E, Dalekos G, Kapatais A, et al. Changes in the use of direct acting antiviral (s) (DAA) in the treatment of chronic hepatitis C (CHC) patients in clinical practice. HERACLIS: a Hellenic multicenter real-life cohort clinical study. Hepatology. 2017; 66: 603A- 4A.
- Manolakopoulos S, Sevastianos V, Triantafyllou K, Goulis I, Cholongitas E, Oikonomopoulou M, et al. Sofosbuvir/Velpatasvir (SOF/VEL)+/-Ribavirin (RBV) in the Treatment of Genotype 3 (GT3) Chronic Hepatitis C (CHC) Patients. Heraclis: A Hellenic Multicenter Real-Life Cohort Clinical Study. Hepatology. 2018; 68: 378A-9A.
- Ticehurst JR, Hamzeh FM, Thomas DL. Factors affecting serum concentrations of hepatitis C virus (HCV) RNA in HCV genotype 1-infected patients with chronic hepatitis. Journal of clinical microbiology. 2007; 45: 2426-33.
- Thomas DL, Astemborski J, Vlahov D, Strathdee SA, Ray SC, Nelson KE, et al. Determinants of the quantity of hepatitis C virus RNA. The Journal of infectious diseases. 2000; 181: 844-51.
- Hagiwara H, Hayashi N, Mita E, Naito M, Kasahara A, Fusamoto H, et al. Quantitation of hepatitis C virus RNA in serum of asymptomatic blood donors and patients with type C chronic liver disease. Hepatology. 1993; 17: 545-50.