
Research Article
J Hepat Res. 2025; 9(1): 1053.
Effects of Hepatitis E Virus-HIV Co-Infection on Haematological and Coagulation Parameters among at Risk Population in Ekiti State, Nigeria
Oshobugie BN¹*, Oluboyo AO¹, Orhe OG³, Osazuwa F², Fasakin KA¹ and Oluboyo BO¹
¹Department of Medical Laboratory Sciences, College of Medical and Health sciences, AfeBabalola University, Ekiti State, Nigeria
²Department of Medical Laboratory Sciences, Edo University, Iyamho, Nigeria
³Department of Family Medicine, Delta State University Teaching Haospital, Oghara, Delta State, Nigeria
*Corresponding author: OSHOBUGIE Blessing Nene, Department of Medical Laboratory Sciences, College of Medical and Health sciences, AfeBabalola University, Ekiti State, Nigeria Tel: +2348057432081; Email: blessinojetu@gmail.com
Received: June 30, 2025 Accepted: July 22, 2025 Published: July 24, 2025
Abstract
Background: HIV-infected patients encompass immunological, epidemiological, and clinical characteristics that can modify the pathogenesis of Hepatitis E virus (HEV); including but not limited to affecting the haematological and coagulation of those co-infected.
Aim: This study is aimed at determining the haematological and coagulation profile (Prothrombin time and activated partial thromboplastin time) among HIV patients co-infected with HEV.
Methods: Three hundred and thirty subjects (330) were recruited after providing informed consent from two tertiary hospitals (Federal Medical Centre, Iddo and Afe Babalola Multisystem specialist Hospital) in Ekiti state, south western, Nigeria. One hundred and eighty (180) HIV infected subjects comprising HAART naïve (30) and HAART treated (150) and one hundred and fifty (150) non-HIV infected subjects (control) were included in this study. Blood samples were analyzed for HEV IgG, IgM by ELISA and were further analyzed for HEV RNA using an in house PCR with commercially prepared primers.
Results: The overall prevalence of HEV among HIV infected subjects was 4/180 (2.2%). HEV was only successfully detected using HEV RNA QPCR. Haematocrit (HCT) levels was significantly lower in HAART-Naïve subjects than in HAART-Treated subjects. Subjects co-infected with HEV and HIV had their prothrombin time tests (PT), as well as activated partial thromboplastin time test (APTT) raised above normal ranges and was significantly higher than values obtained among the healthy controls.
Conclusion: There is a significant presence of HEV infection in our study area with resultant haematological and coagulation effects.
Keywords: HEV; HIV; HAART; PT; PTTK
Introduction
Hepatitis E virus (HEV) infection is the most common cause of acute hepatitis in the world, belonging to the Hepeviridae family [1]. Despite being an important cause of hepatitis and increasing knowledge on the HEV, the origin of the HEV remains obscure [2].
The HEV is a small non-enveloped virus, 27-34 nm in diameter, with a single-stranded positive sense ribonucleic acid (RNA) genome [3]. Among the eight distinct HEV genotypes that have been identified in the Orthohepevirus A species, HEV1, HEV2, HEV3, and HEV4 are able to infect humans. Humans are the main reservoir of HEV1 and HEV2, and any transmission from animals to humans for HEV1 and HEV2 has not yet been reported. The epidemics of HEV1 and HEV2 develop periodically in several regions of Asia, Africa, Mexico, and the Middle East [4].
Human immunodeficiency virus (HIV) and other viral hepatitis (Hepatitis E, B and C virus) co-infections are currently the most documented viral infections of global health importance affecting the world and more importantly the African populace as the prevalence of these chronic illnesses remain high in the sub-Saharan region [5]. HIV infection can lead to abnormalities in complete blood count (CBC) parameters, reflecting the impact of the virus on the hematopoietic system, the body's system for producing blood cells [6]. Coagulation issues frequently arise as complications in individuals with HIV infection [7]. While highly active antiretroviral therapy (HAART) has reduced HIV-related mortality, it has also led to an increase in coagulation abnormalities [8]. This study aim to determine the effect of HIV-HEV co-infection on the haematological and coagulation parameters of HEV infected individuals in Ekiti State, Southwestern Nigeria
Methodology
Study Design
This cross-sectional descriptive study was carried out in two selected tertiary healthcare institutions in Ekiti State Nigeria, the Federal Teaching Hospital, Ido-Ekiti, and ABUAD Multisystem Hospital, Ado-Ekiti.
Study Area
Ekiti State is located in southwestern Nigeria, bordered to the North by Kwara State with 61 km, to the Northeast by Kogi State with 92 km, to the South and Southeast by Ondo State, and to the West by Osun State for 84 km. Named for the Ekiti people—the Yoruba subgroup that make up the majority of the state's population. Ekiti State was carved out from a part of Ondo State in 1996 and has its capital at Ado-Ekiti. According to the National Population Commission of Nigeria census of 2006, Ekiti state has a total population of 2.21 million with sixteen local government. It was estimated that the state has 3, 592, 200 million people as at 2022.
Study Population and Data Collection
This study included One hundred and eighty HIV-infected patients (30 HAART-Naïve and 150 HAART treated), also One hundred and fifty (150) apparently healthy HIV-negative individuals were included as controls.
Sample size calculation: The minimum sample size was calculated by single proportion formula [10].
N = Z²PQ/d²
Where,
N = the minimum sample size
Z = standard normal deviate at 95% confidence level (=1.96)
P = Estimated prevalence of positive HEV in Ekiti State 6.1%, according to a previous study in South-Western, Nigeria [9].
N =76
However, the sample size used for this study was one hundred and eighty (180) HIV-infected subjects to increase the accuracy of the study.
A structured questionnaire was administered to the study participants to gather information on socio-demographics, knowledge of Hepatitis E and HIV, potential risk factors, symptoms, travel and medical histories.
Ethical Approval
Ethical approvals were obtained from the two tertiary hospitals where patients were recruited for this study with protocol numbers ERC/2023/12/14/10538 and ABUADHREC/26/04/2024/384. Written/verbal informed consent was received from subjects before inclusion in this study.
Sample Collection
10mls of venous blood was collected from each consenting subject. 4.5mls was dispensed into well-labeled sterile plain bottles which was separated into serum and packed cells after spinning at 3,000 revolutions per minute (RPM) for 10 minutes. The serum samples were collected and stored in appropriately labeled cryovials, transported to the laboratory on ice pack for storage at -70ºC until analyzed. 3.5 mls was dispensed into EDTA container for CBC, HIV Confirmation test, detection of HEV. The same was done for all control subjects used for this study.
Sample Analysis
HEV IgG/IgM Antibody detection: The Biopanda HEV IgG/IgM Rapid test cassette was used for the detection of IgG/IgM antibodies to Hepatitis E virus among study participants. The Biopanda HEV IgG/ IgM Rapid test cassette is a qualitative membrane-based immunoassay for the detection of HEV antibodies in serum or plasma.
HEV IgG/IgM ELISA: The serum samples were also screened using HEV-IgG/ IgM ELISA Kit (Bioss, Woburn, MA, USA) following the manufacturer's instructions. Briefly, 10 μl of serum sample along with 100 μl of sample dilution buffer was added into the wells of an ELISA plate. The optical density was measured at the wavelength of 450 nm using AMP Micro Plate Reader (Agilent, Santa Clare, CA, USA). The positive and negative results were declared based on absorbance as A > 0.8 and A < 0.1.
HEV RNA (PCR) Detection: The samples were carefully sorted out and 200μl of each sample was pipette into the commercially prepared microtitre plate. It was mixed and taken to the auto-extraction unit and with the aid of SMART 32, an auto extractor, the samples were extracted. 20μl of the supernatant was added to the already prepared primer and taken to the automix room for amplification.
All PCR protocol was carried out on Exicylcer 96 PCR platform (Bioneer, South Korea), using two different PCR assays: a one-step RT-nested PCR and a one-step RT-semi-nested PCR assay with primers lying in the ORF 1 and ORF 2 region of the HEV genome, respectively. Positive and Negative controls where included all batch of a PCR protocol (Table 1).

Table 1: PCR Primers.
Exi Diagnosis software was used to analyze the test results based upon the Ct (threshold cycle) value.
HIV testing by CDC serial algorithm: here, one drop each of whole blood and buffer is placed on Determine test kit. It is allowed to run for 15 minutes. If the result is positive, another test kit, usually Unigold is tested and finally, a tie breaker is introduced, depending on the result.
HIV TESTING BY DIAZON ELISA KIT: The principle of HIV confirmation by Enzyme-Linked Immunosorbent Assay (ELISA) involves coating a plate with specific HIV antigens, blocking unoccupied sites, and adding patient serum or plasma. If HIV antibodies are present in the sample, they bind to the antigens.
Complete Blood Count using MINDRAY Haematology Analyzer
Principle: Mindray Haematology Analyzer operates on a principle that integrates impedance technology for sizing and enumerating blood cells, flow cytometry for distinguishing white blood cell types, and light scatter technology for assessing cell morphology, thereby facilitating a comprehensive diagnostic analysis of blood cell parameters.
Coagulometry: Commercial PT and APTT test kit manufactured by Diagnostic Reagents Limited (Oxfordshire, United Kingdom). Prothrombin time was ascertained by delivering 0.1 ml of plasma into a glass tube placed in a water bath with 0.1 ml of thromboplastin and calcium (a saline brain extract containing tissue factor and a lipoprotein). Activated partial thromboplastin time in kaolin was done by mixing equal volumes of the phospholipids reagent and the kaolin suspension. The test was carried out in duplicate for both controls and the study subjects and the mean value for each obtained. Deficiency of factors and fibrinogen will result in prolonged clotting time.
Statistical Analysis: Data analysis was performed using SPSS v. 18. Statistical significance was expressed at values <0.05.
Results
Using Rapid HEV IgG/IgM test kits and ELISA, The One hundred and eighty HIV-infected subjects (Both HAART –naïve and HAARTtreated) where all negative for Hepatitis E virus antibodies. Further testing of serum samples for HCV RNA QPCR showed a positive detection report of 4 out of 180 HIV infected subjects with an overall HEV positivity rate of 2.2% (Table 2). The prevalence of HEV RNA detection were found in two each of male and female subjects (Table 3).
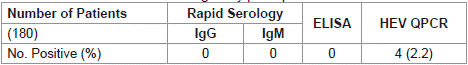
Table 2: Prevalence of HEV among study participants.
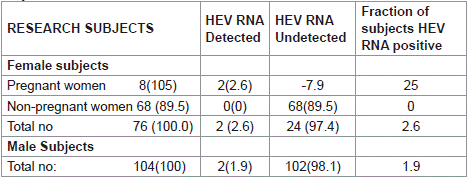
Table 3: HEV-RNA Qualitative findings among the HIV-HEV Seropositive
subjects.
Table 4 shows the comparism of mean haematological parameters of HAART-Naïve and HAART Treated subjects. Haematocrit (HCT) was lower in HAART-Naïve subjects than in HAART-Treated subjects and it was statistically significant with p value of 0.0026. Other haematological parameters showed no significant difference.
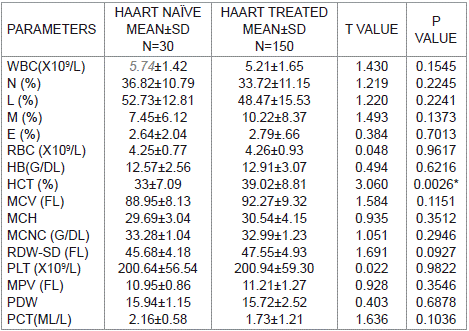
Table 4: Comparison of mean haematological parameters of HAART-naïve and
HAART- treated research subjects.
Table 5 shows the measured coagulation parameter findings among HEV-HIV Infected subjects. Four (4) research subjects were co-infected for HEV and HIV with their prothrombin time tests (PT), as well as activated partial thromboplastin time test (APTT) raised above normal ranges and was significantly higher than values obtained among the healthy controls.

Table 5: Coagulation profile of HIV-HEV co-infected subjects.
Table 6 Show the compares of all parameters for HAART treated (third trimester) pregnant women and HAART-naïve subjects. The mean total white cells count for pregnant subjects on HAARTwas lower when compared to the mean total white cells count of HAART-Naïve subjects (P=0.0003). likewise, total red cell count (RBC), Haematocrite (HCT), mean cell volume (MCV), mean cell haemoglobin count (MCHC), Red cell distribution width (RDW), Platelet count (PLT), Mean platelet volume (MPV), Platelet distribution width (PDW), Plateletcrit (PCT), Haemoglobin (HB), Viral load and CD4 count were lower when compared with HAARTnaïve subjects with a significant p value of <0.0001. The mean cell haemoglobin (MCV) is higher in the pregnant subjects than in the HAART-naïve subjects and is significant at p<0.0001.
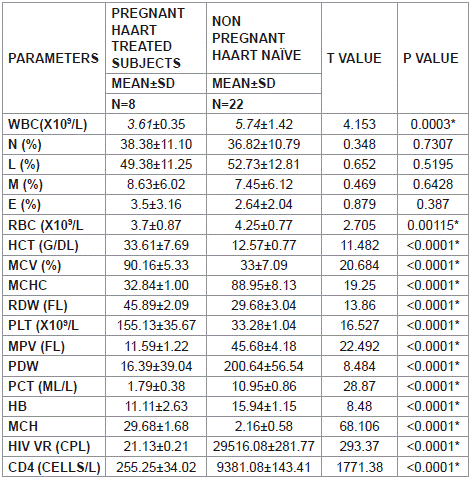
Table 6: Correlations of parameters for pregnant women in their third trimester
and HAART-naïve subjects.
Discussion
The prevalence of HEV-HIV co-infection was investigated in this study alongside with some selected haematological, and coagulation parameters. The prevalence of HEV among HIV infected subjects in our study area was 4/180 (2.2%). This study uncovered the existence of an occult HEV infection among study participants, as only the HEV QPCR assay revealed the presence of HEV among the population studied. This observation calls for the utilization of molecular methodology for proper epidemiologic survey of HEV in our locality. The prevalence report in our study is close to 4.8% HEV prevalence among blood donors in Lagos [10], and 5.5 % in Ogbomoso, Oyo State, Nigeria [11]. The most effective preventative measure for HEV infection is to avoid contact with the source of infection, such as avoiding the consumption of raw/undercooked food where HEV has been isolated or the consumption of unchlorinated contaminated water in low-income countries.
There were no significant difference in HEV prevalence based on gender, this has been proven in some previous studies that HEV prevalence may not be gender related. This finding concurs with previous research done at Osun State, where there were no significant association between gender of subjects and sero-positivity for HEV [12].
There were significant difference in the haematocrite (HCT) of HAART-naïve compared with HAART- treated research subjects p<0.05. HAART-naïve individuals had lower hematocrit (Hct), indicating anaemia that may be caused by chronic inflammation, opportunistic infections, and viral-related implications. This is in agreement with the work done by Obeagu et al. [13], which reviewed the complex interaction between anaemia and HIV, highlighting the impact of chronic inflammation. It is also in tanderm with the report of Adams et al., [14] which explained that HIV-related anaemia results from various factors, including direct viral effects on bone marrow, chronic inflammation, nutritional deficiencies, and adverse effects of Antiretroviral Therapy (ART). According to Vishnu and Aboulafia, [15] direct viral effects on haemopoiesis and the chronic inflammatory state indicated by HIV contributed to the development and exacerbation of anaemia [16]. Anaemia in HIV Patients is associated with a range of clinical implications, including increased morbidity and mortality. Anaemia correlates with accelerated disease progression, higher rates of infections and a decline in overall immune function [16].
Prothrombin time and activated partial thromboplastin time were significantly higher among HEV-HIV co-infected subjects when compared to healthy controls used for this study. It has been established in some previous studies that coagulation parameters are prolonged in HIV infection [17,18]. In HIV infection, haemostatic disorders occur as a result of acquired deficiency of anticoagulant proteins such as protein C and protein S, heparin cofactor II, and increased concentrations of coagulation and fibrinolytic markers [19].
In this study, hematological parameters varied significantly in HIV-positive pregnant subjects in their third trimester compared to HAART – naïve subjects. Significant decrease was recorded for WBC, RBC, MCHC, HB, MPV, PCT, PDW, HIV viral load, and CD4 count of pregnant women in their third trimester compared with HAARTnaïve subjects, where as significant increase was reported for HCT, MCV, and PLT. Hematological parameters, such as complete blood count (CBC) provide information on the impact of HIV on red and white blood cells and platelet counts, indicating possible anaemia, leukopenia, or thrombocytopenia. Elevated mean corpuscular volume (MCV) indicated macrocytosis which may be attributed to vitamin deficiencies or medications of the pregnant women which concurs with the work of Nye et al. [20] who examined the effect of lamivudine and emtricitabine on mean corpuscular volume (MCV) in HIV patients, indicating that these medications can lead to macrocytosis.
Decreased mean corpuscular hemoglobin concentration (MCHC) may suggest hypochromic anaemia, consistent with known haematological abnormalities in HIV patients, and is consistent with the work of Kaudhaet al. [21]. Thrombocytopenia, evidenced by low platelet count, among HAART – naive was attributed to bone marrow suppression or immune system destruction of red blood cells [22]. Elevated mean platelet volume (MPV) among the HAART-naives indicated platelet activation, while increased red cell distribution width (RDW) among the HIV positive pregnant subjects suggested size variability among erythrocytes, characteristic of underlying pathology like ineffective erythropoiesis [22]. The decreased WBC among the HIV positive pregnant subjects supports the hypothesis that CBC profiles may serve as early predictors of compromised immune systems in HIV-positive individuals, aiding timely treatment adjustments [23]. Leucopenia is frequently seen in HIV patients and is predominantly due to lymphopenia and occasionally, reduced production of granulocytes and monocytes. Due to the inhibition of granulocyte progenitors, leucopaenia may also results and can result from concurrent infections, immune – mediated or therapy related factors [24].
Conclusion
In conclusion, the prevalence of HEV among HIV infected subjects in this study was 2.2 %. Distribution among gender was not skewed to any particular gender. HIV infected subjects that were HAART naïve significantly had a lower hematocrit value. Coagulation parameters (PT and APTT) was significantly higher in HEV-HIV coinfected subjects as compared to the controls.
Footnote
The data used for this study is part of the doctoral thesis of the first author.
Acknowledgement
Authors appreciate with thanks the management of FMC Iddo and ABUAD Multisystem hospital for providing approval for inclusion of their patients. Thanks also goes to all participants for providing consent for inclusion in this study
Funding
Authors didn’t receive any funding from any agency for carrying out this research. This research was funded by authors.
References
- Aslan AT, Balaban HY. Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment. World J Gastroenterol. 2020; 26: 5543-5560.
- Balayan MS, Andjaparidze AG, Savinskaya SS, Ketiladze ES, Braginsky DM, Savinov AP, et al. Evidence for a virus in non-A, non-B hepatitis transmitted via the fecal-oral route. Intervirology. 1983; 20: 23–31.
- Guerra JAAA, Kampa KC, Morsoletto DGB, Junior AP, Ivantes CAP. Hepatitis E: A Literature Review. J ClinTranslHepatol. 2017; 5: 376–383.
- Aggarwal R. Hepatitis E: Historical, contemporary and future perspectives. J Gastroenterol Hepatol. 2011; 26: 72–82.
- Singh KP, Crane M, Audsley J, Avihingsanon A, Sasadeusz J, Lewin SR. HIV-hepatitis B virus coinfection: epidemiology, pathogenesis, and treatment. AIDS. 2017; 31: 2035-2052.
- Agrawal D and Sarode R. Complete Blood Count or Complete Blood Count with Differential: What’s the Difference? The American Journal of Medicine. 2017; 130: 915-916.
- Ojerinde OA, Ojo S, KS Udewena UL and Oladeji SJ. A cross-sectional study on the prevalence of HIV and hepatitis B virus co-infection among students of a tertiary institution in Ekiti State, Southwest Nigeria. Pan African Medical Journal. 2023; 44: 7.
- Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A & Sabin CA. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. Journal of Acquired Immune Deficiency Syndromes (JAIDS). 2018; 79: 166- 178.
- Adesina OA, Shodunke OC, Adedara OO, Oluyege AO. Hepatitis E virus immunoglobulin M (IgM) and associated risk factors in Southwest Nigeria. Ife Journalof Sciencevol. 2021; 23: 51-62.
- Uche EI, Olowoselu OF, Ekwere TA, Ismail AK & Akinbami AA. Prevalence of Hepatitis E Virus Infection among Blood Donors seen at the Lagos State University Teaching Hospital, Nigeria. Nigerian Journal of Haematology. 2023; 4: 48-56.
- Olawole OE. Detection of Hepatitis E virus among HIV infected individuals in Ogbomoso, South-western Nigeria. Br. J. Virol. 2015; 2: 62–67.
- Osundare FA, Klink P, Akanbi OA, Wang B, Harms D, Ojurongbe O, et al. Hepatitis E virus infection in high-risk populations in Osun State, Nigeria. One Health. 2021; 13: 100256.
- Obeagu, Emmanuel Ifeanyi PhDa, Obeagu, Getrude Uzoma BNScb, Ukibe, Nkiruka Rose PhDc; Oyebadejo, Samson Adewale PhDMD. Anemia, iron, and HIV: decoding the interconnected pathways: A review. Medicine. 2024; 103: p e36937.
- Adam I, Ibrahim Y & Elhardello O. Prevalence, types and determinants of anemia among pregnant women in Sudan: a systematic review and metaanalysis. BMC Hematol. 2018; 18: 31.
- Vishnu P, Aboulafia DM. Haematological manifestations of human immune deficiency virus infection. Br J Haematol. 2015; 171: 695-709.
- Nguyen H and Patel S. CBC parameters and ART response in HIV. AIDS Treatment and Management. 2018; 22: 175-190.
- Seemitr Verma, Ruchee Khanna, Vishwapriya Godkhindi, Anjali Vijay S, Shashidhar V, SM Zeeshan. Study of Coagulation parameters in HIV patients and its relation to CD4 counts and ART status. Research Journal of Pharmacy and Technology. 2023; 16: 489-484.
- Baker J V. Chronic HIV disease and activation of the coagulation system. Vol. 132, Thrombosis Research. Elsevier Ltd; 2013: 495–499.
- Kuller LH, Tracy R, Belloso W, Wit SD, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection, PLoS Medicine. 2008; 5: e203.
- Nye, Clemency, Joanna Latimer, and Mark Gompels. “Macrocytosis Associated with Lamivudine and Emtricitabine Use in Patients with HIV.” J AIDS Clin Res. 2020; 12: 827.
- Kaudha R, Amanya R, Kakuru D, Muhumuza Atwooki R, MutebiMuyoozi R, Wagubi R, Muwanguzi E, Okongo B. Anemia in HIV Patients Attending Highly Active Antiretroviral Therapy Clinic at Hoima Regional Referral Hospital: Prevalence, Morphological Classification, and Associated Factors. HIV AIDS (Auckl). 2023; 15: 621-632.
- Getawa S and Adane T. Coagulation Parameters in Human Immunodeficiency Virus-Infected Patients: A Systematic Review and Meta-Analysis. AIDS Research and Treatment. 2022; 2022: 6782595.
- Lohse N and Obel N. Upate of Survival for persons with HIV Infection in Denmark. Ann Intern Med. 2016; 165: 749-750.
- Smith J. Importance of complete blood count and HIV serotyping in managing individuals with HIV. Journal of Clinical Hematology. 2019; 6: 260-275.