
Review Article
Austin J HIV/AIDS Res. 2024; 10(1): 1057.
Tuberculosis Diagnosis in Patients Co-Infected with HIV: A Review
Priyanka Gupta1*#; Abhishek Gupta2#; Kaleshwar Prasad Singh3
¹Department of Clinical Hematology, King George’s Medical University, India
²Department of Physiology, King George’s Medical University, India
³Department of Microbiology, King George’s Medical University, India
*Corresponding author: Priyanka Gupta MSc, MPhil, PhD Department of Clinical Hematology, King George’s Medical University, Lucknow, India. Tel: +91-7668825683 Email: guptapg1985@gmail.com
#These authors have been equally contributed to this article.
Received: June 13, 2024 Accepted: July 09, 2024 Published: July 16, 2024
Abstract
Co-infection with Human Immunodeficiency Virus (HIV) and Tuberculosis (TB) interacts in fundamentally significant ways. Pathophysiological, clinical, and epidemiological evidence all support this connection. HIV-positive and HIV-negative TB patients differ in a few ways that could affect practical diagnostics. When immunosuppression increases, TB becomes more transmissible in nature and more challenging to diagnose using standard diagnostic methods. Better diagnostic methods should be taken into account as TB control measures since TB rates are rising in areas where HIV is widespread. It is necessary to create more approachable methods that can be modified for usage in high-burden and low-income nations. This review focuses on the challenges associated with detecting co-infection between HIV and TB, giving an update on existing diagnostic methods and describing potential developments in light of the HIV pandemic.
Keywords: HIV; Tuberculosis; Co-infection; IRIS; Diagnosis; Immunosuppression
Introduction
Tuberculosis (TB) and Human Immunodeficiency Cirus (HIV)/ acquired immune deficiency syndrome (AIDS) are the two most prevalent infectious illnesses in low-resource countries [1]. TB is also the leading cause of death for persons with AIDS, taking the lives of one in three of these patients [2]. TB is the second most common deadliest infectious disease in the world, after COVID-19, and ahead of HIV and AIDS. In 2022, 10.6 million cases of TB are expected to occur worldwide. The incidence of TB has significantly increased worldwide as a result of HIV infection. In nations with high rates of HIV prevalence, TB is the leading cause of mortality [3]. This is due to HIV-related impairments in cell-mediated immune responses, which lead to increased susceptibility to TB and a quick conversion of latent TB to active illness. It will be challenging to stop the spread of TB and lower mortality in places where HIV infection is common without improved TB diagnostic methods and practical implementation strategies. Improved diagnostic test development and application are desperately needed to help TB control in HIV-positive communities.
Treatment for coinfected persons is advised regardless of CD4+ cell count because prompt ART initiation improves survival rates for all HIV-positive individuals, including those co-diagnosed with TB. This strategy highlights the value of early intervention and the critical role that antiretroviral therapy (ART) plays in improving outcomes for this vulnerable population [4]. Still up for contention, though, is the appropriate time to start ART. Updated recommendations for starting antiretroviral therapy (ART) in adults and adolescents are based on the WHO clinical stage.
Revised NACO Guidelines [4]
For the management of HIV infection in areas with limited resources, ART is now widely available. But many people who require ART start treatment too late, frequently having already developed clinically severe TB when they seek medical attention. The concurrent use of ART and Antitubercular Treatment (ATT) is essential for many coinfected individuals because it significantly increases survival [5,6]. According to an earlier study, high levels of coverage and compliance are essential for the best possible administration of ART to treat HIV infection and maintain immunity, hence preventing TB [7]. However, co-administration presents a number of management issues, such as the possibility of medication interactions, the occurrence of drug-related toxicities that overlap, and the development of TB associated immune reconstitution Inflammatory Syndrome (IRIS).
Immune Reconstitution Inflammatory Syndrome
TB associated IRIS is the term for any TB manifestation identified when a patient is receiving ART [8,9]. M.tb is the infectious precipitant of IRIS that is most frequently reported. The most typical presentation of TB-IRIS is a paradoxical disease that strikes people who are responding well to anti-TB medication. It typically shows up in the first two months and frequently in the first three weeks of ART. TB detected during ART ought to be referred to as ART-associated TB [10]. Patients generally have increasing chest radiographic appearances along with recurrence or worsening of constitutional symptoms, such as high fever and node enlargement that may become suppurative [11].
TB-IRIS have been classified into two disease patterns based on INSHI diagnostic criteria [12]. Initially, Paradoxical disease, often known as paradoxical IRIS, is characterized by a known infection that worsens even after treatment with Antiretroviral Therapy (ART). This could be a reaction to the antigens of non-viable infections or a reaction to live pathogens. Second, unmasking disease, also known as unmasking IRIS, is an immunological reaction to a virus that wasn't causing obvious disease prior to the introduction of ART. Viable pathogens are typically involved in uncovering disease [12].
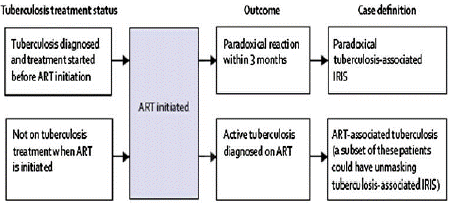
INSHI diagnostic criteria of TB-IRIS [12]
INSHI diagnostic criteria of TB-IRIS [12]
Prior to the widespread emergence of HIV infection, an IRIS-like paradoxical inflammatory response was known to occur in some patients treated for TB [13,14]. Patients who are coinfected with M.tb and HIV may also develop IRIS that manifests as cervical tuberculous lymphadenitis (Figure 1), intracranial tuberculomas, cutaneous lesions, peritonitis, epididymitis, bowel perforation, or granulomatous nephritis.
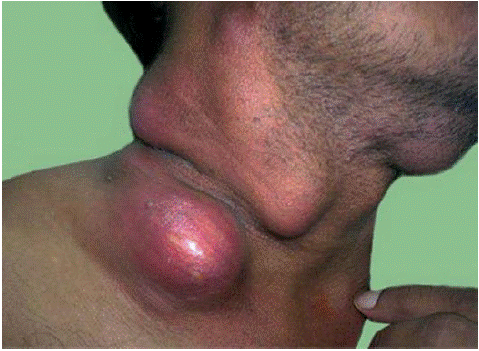
Figure 1: Cervical Lymphadenitis in Patient with TB-IRIS.
Healthcare systems are constantly under pressure from both HIV and TB combined load. First of all, because HIV and TB both exacerbate symptoms of one other, co-occurring conditions dramatically raise fatality rates. Coping with TB and HIV at the same time poses difficulties and complicates the treatment plan due to the combination of pharmacological toxicities, a substantial medication regimen, and possible drug interactions [15]. Making a diagnosis can be difficult and sometimes involves specialized testing and understanding because HIV-positive individuals frequently have different symptoms and impaired immune systems. The purpose of this review is to clearly declare that improved diagnostic tests for TB are necessary and to provide an overview of the approaches and emerging technologies being investigated to address this need.
The Diagnostic Dilemma
In HIV-positive patients, the diagnosis of TB may be challenging due to a variety of factors, including an increased frequency of sputum smear negativity (up to 40% in pulmonary cases confirmed by culture), unusual radiographic findings, absence of classic granuloma formation, and negative tuberculin skin test results. Additionally, in resource-poor areas where access to histopathology and advanced imaging testing is either limited or nonexistent, the elevated frequency of extrapulmonary TB in HIV patients poses a unique diagnostic difficulty [16]. Numerous HIV-related pulmonary disorders might resemble TB and complicate the diagnosis. Patients are frequently started on anti-TB therapy incorrectly based only on clinical presentation because there is no accurate, quick test for smear-negative TB in adults and children. This means that we frequently miss the correct diagnosis. Because TB's clinical presentation is mimicked by many other opportunistic illnesses encountered in HIV patients, treating these diseases prematurely increases the risk of severe treatment responses, drug interactions, and drug resistance [17]..
Early detection and prompt treatment initiation are necessary for an efficient TB control program. The prognosis of the disease for each individual as well as the community and the pace of reproduction of the TB epidemic are significantly impacted by delays in diagnosis [18-20]. As a result, the interval between the time of first encounter and diagnosis is four times longer. Healthcare professionals at HIV treatment facilities may fail to diagnose TB or delay doing so due to the high rates of sputum smear negative.
In order to detect HIV-associated TB more rapidly, it is necessary to develop more effective assays that utilize active case finding and condensate clinical algorithms. They may be more sensitive, produce findings more quickly, or be easier to use, which could shorten the time between the start of symptoms and the start of medication. Microscopy and culture could be replaced with a straightforward point-of-care test that combines these criteria and provides a confirming diagnosis during the initial clinic visit. Naturally, both HIV-positive and HIV-negative populations would be significantly impacted by such a test.
Surprisingly high rates of HIV-related Multidrug Resistant-TB (MDR-TB) have been discovered in various developing-nation settings [21,22]. Improved MDR detection is therefore also required. Rapid methods to identify extremely resistant M.tb strains are crucial, as evidenced by recent reports of deadly outbreaks of extensively drug-resistant-TB (XDR-TB) [23]. Better targeting of preventive medication could result from a more accurate identification of latent infection with M.tb in HIV-positive individuals, with stronger negative and positive predictive values than current tubercular skin tests.
Diagnosis of TB in HIV Infected Patients
A high index of suspicion is crucial for TB diagnosis. HIV co-infection impairs the effectiveness of the acid-fast bacilli sputum smear and complicates the clinical presentation and diagnosis of active TB. The most popular TB diagnostic technique in environments with limited resources is the AFB sputum smear, which is also the gold standard for TB diagnosis in low- and middle-income nations [24,25].
In HIV-endemic areas, the low clinical sensitivity of the technique in HIV-positive persons and the logistical challenge of providing high-quality microscopy access in resource-constrained settings are the two main constraints/ limitations of microscopic diagnosis of TB. Low sensitivity in microscopy is one of its primary limitations. Sputum microscopy has an average sensitivity of less than 60% for detecting pulmonary TB in immunocompetent populations [26-28]; in HIV-positive individuals, this sensitivity is much lower [29]. This raises the risk of TB transmission to other patients, staff, homes, and community members in addition to the patient's own risk of morbidity and mortality.
Limited availability of high-quality microscopy services is another issue. Conditions that are not ideal frequently make the test's limited sensitivity worse. A study found that in Tigray region of northern Ethiopia with high HIV infection rates, the percentage of patients identified by microscopy can range from 20% to 35% [30]. Because of the expense, sputum cultures are not frequently performed in settings with low resources, even if they are beneficial for patients with smear-negative diseases. The timely detection of TB in immunocompromised populations is crucial for both disease control and patient care. It is obvious that it is fatal to wait to diagnose TB in people who also have HIV. Compared to their peers with smear positive disease, patients with smear negative disease frequently have worse treatment outcomes and higher mortality rates [31].
The diagnostic cornerstone for pulmonary TB is the chest radiograph. According to study published in 1995, radiographic patterns of HIV-positive individuals with higher CD4 T-cell counts (e.g., >200 cells/mm3) typically show signs of reactivation illness, including upper lobe infiltrates with or without cavities [32]. Lower lobe infiltrates and intrathoracic lymphadenopathy are indicative of a main disease pattern when the CD4 T cell count is less than 200 cells/mm3. In assessing an HIV-positive patient exhibiting symptoms indicative of TB, a high index of suspicion must be maintained because chest radiographs may show normal in 7–14% of instances [33,34].
Prior to this, Quantiferon-TB Gold (QFT) and the T-SPOT.TB tests are the two most reliable assays available for detecting TB infections. Since, they gauge the release of interferon gamma from cells in vitro. These tests are collectively referred to as interferon-gamma release assays, or IGRAs. A third of the world's population has been shown to be sensitized to M.tb by immunological testing including TST and interferon gamma release assays (IGRAs). Hence, it is predicted that approximately two billion people are unmaskly infected with M.tb. These people do not exhibit any clinical symptoms but are at danger of developing overt clinical disease in the future, especially if they also have HIV co-infection [35].
Diagnostic Testing for The Identification of Drug Resistance and HIV-Associated TB
In many countries, the diagnosis of HIV-associated TB includes smear microscopy, mycobacterial culture, NAATs and urine LAM assay (Table 1).
Diagnostic Test
Time period
Benefits
Draw backs
Smear microscopy
Conventional
Same day
Is a quick and affordable method
Insensitive
LED
Same day
Greater sensitivity compared to traditional microscopy methods
----
Mycobacterial culture
Culture with DST
2-3 weeks
Compared to solid culture, liquid culture is more sensitive
Necessitate level 3 biosafety laboratory, and outcomes take time
NAATs
Xpert MTB/RIF
Same day
Rapid diagnosis times, great sensitivity for smear-positive TB, and simultaneous detection of RIF
Too expensive and unable to differentiate between living and dead Bacilli
LAMP
Same day
Is affordable
Requirements for infrastructure are crucial
First-line LPA
1-2 days
Identification of resistance to INH and RIF
Affordability and implications for resources
Second-line LPA
1-2 days
Fluoroquinolone and second-line injectable medication detection
Affordability and implications for resources
Antigen detection test
LAM
Same day
Quick diagnosis
Insensitive
Table 1: An overview of diagnostic procedures for HIV-related TB and treatment resistance detection.
Bacilloscopy Methods
Under a microscope, a stained sputum smear is examined using the Ziehl-Neelsen (ZN) method to diagnose TB in several countries. Visible under a microscope, sputum smears can reveal the presence of acid-fast mycobacteria. Ziehl-Neelsen-stained slides are examined under a light microscope as the most often used technique. For the diagnosis of TB in endemic areas, direct microscopy now offers the benefits of being a quick, low-cost method with excellent specificity. As reported in 2001, AFB microscopy is quick and cheap, but its sensitivity is only 40–60% [36]. Three sputum specimens should be taken from each patient suspected of having pulmonary TB on three separate days. These specimens should then be cultured for mycobacteria and analyzed for AFB.
Fluorescence microscopy and Auramine-Rhodamine staining are two additional effective modern diagnostic methods [37,38]. Fluorescence microscopy is superior in this aspect because it is more sensitive and enables quick screening of a large number of smears. Fluorescence microscopy has several key limitations, one of which being its high cost. However, using fluorescent Light-Emitting Diodes (LEDs) in place of traditional fluorescent light sources can drastically reduce costs. Consequently, switching to LED microscopy from traditional fluorescent light sources is one of the WHO's key recommendations for smear microscopy.
Mycobacterial Culture
One crucial conventional test for TB diagnosis is still M.tb culture in liquid or on solid agar. Liquid culture is more costly than solid culture. Liquid culture, however, is more delicate and quicker. 5,000–10,000 bacilli mL-1 are required for microscopy, whereas 100 bacilli mL-1 of sputum can be detected with this method [39]. Material for additional identification and drug susceptibility testing is also provided. Nevertheless, it takes a long time- between three and eight weeks- to see the results. Although, culture has 80% sensitivity, it can take four to eight weeks to diagnose a patient using culture.
The strains used in this diagnostic test are available for Drug Susceptibility Testing (DST) and genotyping, which can be used to identify outbreaks or transmission episodes. These are significant advantages. Mycobacterial culture has significant drawbacks, including the requirement for infrastructure to support suitable biosafety level 3 facilities and quick sample transportation to the lab.
Nucleic Acid Amplification Tests (NAATs)
The highly sensitive detection of paucibacillary forms of TB is a possible benefit of NAATs. Molecular methods like the Xpert MTB/RIF and the Loop-mediated isothermal Amplification Test (LAMP) are currently crucial in the diagnosis of TB [40]. These approaches use real-time polymerase chain reaction (PCR) or isothermal amplification to quickly detect the presence of both MTB and mutations linked to Rifampicin (RIF) resistance at the same time.
Loop-mediated Amplification Test
The current NAAT temperature-independent DNA amplification technique is the TB-loop-mediated isothermal amplification test assay. This test has a significant financial benefit because the results are shown in an easily readable visual display [41].
Urine lipoarabinomannan Lateral Flow Test
The availability of antigen testing is expanding, even in nations with low resources. Urinary Lipoarabinomannan (LAM) testing is the most often used test. A lateral flow technique for the diagnosis of hematogenous disseminated TB uses urine-based detection of the mycobacterial antigen LAM [42]. According to 2016 study, there may be a lower death rate in persons with HIV who use LAM for TB diagnosis [43]. As per previous studies, LAM has a high specificity of 88% to 99% and is particularly sensitive in HIV-positive individuals with low CD4+ cell counts [44,45].
Drug Susceptibility Testing Methods
Traditionally, Drug Susceptibility Testing (DST) and case detection have been the main priorities of disease control initiatives in TB-endemic nations. The majority of poor nations often use solid media for DST, such as Lowenstein-Jensen media (L-J). It usually takes 8 to 18 weeks for results to become available, during which time many individuals with MDR-TB or XDR-TB may have passed away, spread their illness, or both. Many new, quicker methods have been developed recently to detect medication resistance.
The proportion, absolute concentration, and resistance ratio approaches are examples of Drug Susceptibility Testing (DST) methods [46,47]. These four methods were regarded as the gold standard [48] once the BACTEC radiometric system was introduced and modified to perform DST of M.tb [49,50]. These new methods might be classified as phenotypic or genotypic methods.
There are two main processes involved in this process: first, amplification of certain regions of the M.tb genome known to be altered in resistant strains using molecular nucleic acid amplification methods like PCR; second, examination of the amplified products for specific mutations associated with resistance. These methods have a number of benefits, including a shorter Time to Treatment (TTT) (days as opposed to weeks), the ability to apply the organism directly to clinical specimens, a lower risk of biohazard, and the potential for automation. As the most used technique, DNA sequencing of PCR-amplified products has emerged as the gold standard.
In order to evaluate M.tb suppression in the presence of antibiotics, new phenotypic approaches use a variety of technologies to identify early growth indicators. The MGIT system has been thoroughly evaluated for DST of M.tb to first- and second-line drugs, both manually and automatically. Based on the measurement of oxygen consumption, the system shows good concordance with the gold standard proportion method [51,52].
Molecular Diagnostic Methods
The diagnosis of TB has improved dramatically with the advent of numerous Nucleic Acid Amplification (NAA) techniques, including PCR, which has been extensively evaluated for its ability to provide a prompt diagnosis. PCR is a quick, accurate, and sensitive diagnostic method that may identify pulmonary TB in HIV-positive persons with a sensitivity higher than microscopy and a specificity comparable to culture, even in cases with low bacillary burden or early stages of the disease [35]. PCR has a 90% sensitivity for detecting respiratory TB and a 95–99% specificity. Two previously approved commercial NAA techniques are the Amplicor M.tb and the amplified M.tb direct test (AMTD) (Gen-Probe). Both techniques have been approved for the direct diagnosis of M. tuberculi in respiratory specimens that are smear-positive.
When compared to culture and clinical status, these methods produce greater sensitivity and specificity in smear-positive specimens; but, because they produce lower results in smear-negative specimens, they are not helpful as a screen to rule out the disease. Two more recently established methods for diagnosing TB are strand-displacement amplification and real-time PCR. The sensitivity has ranged from 71% to 98% with a nearly 100% specificity [53]. Real-time PCR has two main advantages: it produces results quickly-it takes 1.5-2 hours after DNA extraction and has a lower risk of contamination because the reaction and detection happen in the same tube.
In general, it has been discovered that gene amplification methods are quite sensitive and specific for diagnosing TB straight from clinical specimens. Direct identification of M.tb is rare and requires a lot of time. Sixty percent of specimens that are negative for mycobacterial culture can be confirmed by PCR. The diagnosis of TB affecting the skin, eyes, bones, genitalia, and lymph nodes has also been demonstrated to benefit from PCR. A legitimate fear has been raised over false positive results brought on by contamination in labs and clinics. Proper lab design, stringent specimen collection and processing guidelines, careful reagent handling, and the use of specific blocking agents can all significantly reduce false positive issues.
In a developing country such as India, the establishment of a high-class laboratory requires significant infrastructure facilities and consideration of patient costs. Adopting such approaches shouldn't be discouraged by cost, though, as the cost of primers and reagents has significantly decreased over the last four to five years, which is encouraging given the benefits of speed, sensitivity, and specificity.
The most focus has been paid to Adenosine Deaminase (ADA) detection as an indirect method of diagnosis among non-microbiological diagnostic procedures used for TB. The majority of cells contain the enzyme ADA, yet Tuberculous Pleural Effusions (TPE) have been shown to have higher levels of this enzyme [54]. Because ADA level determination can be done quickly, simply, and affordably using a colorimetric method, it has been hailed as a promising marker.
The early radioactive diagnosis of TB meningitis is achieved through the use of the radioactive bromide partition test. Following a loading dosage, the distribution of bromide ions between serum and CSF indicates the health of the blood-brain barrier. The test has a sensitivity of about 90%. Rapid and accurate identification of M.tb and atypical mycobacteria has been achieved through the use of gas chromatography of mycobacterial fatty acids. When using gas chromatography-mass spectrometry with Selected Ion Monitoring (SIM) to detect tuberculostearic acid in sputum [55,56] and CSF [57], the results showed that the method was slightly less sensitive than traditional culture methods but still more sensitive than conventional microscopy. The usage of this technique is currently uncommon.
Conclusion
Several current technologies- including those related to HIV-associated TB are being developed for better TB diagnosis. Every day, advances in research and innovation have transformed the global landscape of TB diagnosis, treatment, and prevention. Still, the majority of TB patients and susceptible groups at elevated risk of TB transmission lack access to the most recent diagnostic methods. In order to curb the TB pandemic, it is a good idea to move forward with the development of more practical, quick, and sensitive molecular diagnostic assays to detect subclinical HIV co-infection. Modern molecular diagnostic tests are either quick enough or accurate enough to treat HIV co-infected patients, even if the usage of these tests has increased over the last ten years in the detection of TB. The creation of new instruments raises the possibility that they will require pricey technologies that developing nations impacted by the HIV/TB pandemic cannot afford. To assist the discovery of new biomarkers and their translation into clinical applications, more funding is required in the interim.
Author Statements
Author Contribution Statements
PG created the review's framework. After compiling information and data from the literature, PG and AG wrote a summary of the information and created the review manuscript. The review manuscript has been read and approved by all of the authors, including PG, KPS, and AG.
Funding
There is no source of funding for this review that need to be disclosed.
Conflict of Interest
There is no conflict of interest to disclose.
Acknowledgement
The authors would like to express their gratitude to the King George's Medical University ART Center team in Lucknow, India, for their unwavering assistance in gathering data on HIV patients. HIV patients are sincerely thanked by the authors for their involvement.
References
- Tola A, Mishore KM, Ayele Y, Mekuria AN, Legese N. Treatment Outcome of Tuberculosis and Associated Factors among TB-HIV Co-Infected Patients at Public Hospitals of Harar Town, Eastern Ethiopia. A five-year retrospective study. BMC Public Health. 2019; 19: 1658.
- WHO Global Tuberculosis Report. 2019.
- World Health Organization. WHO Report: Global Tuberculosis report (2023). 2023.
- National AIDS Control Organization (NACO) OI Guidelines. Antiretroviral Therapy Guidelines of HIV-Infected Adults and Adolescents Including Post-exposure Prophylaxis. NACO, Ministry of Health & Family Welfare Government of India. 2019.
- Gezae KE, Abebe HT, Gebretsadik LG, Gebremeskel AK. Predictors of time to death among TB/HIV co-infected adults on ART at two governmental hospitals in Mekelle, Ethiopia, 2009–2016: A retrospective cohort study. Ann Infect Dis Epidemiol. 2020; 5: 1049.
- Wondimu W, Dube L, Kabeta T. Factors Affecting Survival Rates Among Adult TB/HIV Co-Infected Patients in Mizan Tepi University Teaching Hospital, South West Ethiopia. HIV AIDS (Auckl). 2020; 12: 157-64.
- Gupta P, Tripathi AK, Jain A, Prasad R, Singh KP, Vaish AK, Misra RP. Effect of IRIS development on survival in HIV-TB patients on antiretroviral therapy among north Indian population. Ind J Comm Health. 2012; 24: 129-33.
- Lawn SD, Wood C, Lockwood DN. Borderline tuberculoid leprosy: an immune reconstitution phenomenon in a human immunodeficiency virus-infected person. Clin Infect Dis. 2003; 36: e5-6.
- Gupta P, Tripathi AK, Jain A, Singh KP, Misra RP. Redefining the role of Tuberculin skin test of TB-IRIS in HIV Positive patients on antiretroviral therapy in North Indian Population. Ind. J Prevent Soc Med. 2012.
- Navas E, Martin-Davila P, Moreno L, Pintado V, Casado JL, Fortun J, Perez-Elias MJ, Gomez-Mampaso E, Moreno S. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy. Arch Intern Med. 2002; 162: 97-9.
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998; 158: 157-61.
- Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis- associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009; 30: 797–810.
- Cheng VC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PC, et al. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002; 21: 803-9.
- Yang H, Liu Q, Wu Y, He K, Zeng Q, Liu M. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome in initiating ART among HIV-Infected patients in China-risk factors and management. BMC Infect Dis. 2024; 24: 5.
- McIlleron H, Meintjes G, Burman WJ, Maartens G. Complications of antiretroviral therapy in patients with tuberculosis: drug interactions, toxicity, and immune reconstitution inflammatory syndrome. J Infect Dis. 2007; 196: S63-75.
- Harries AD, Nylrenda TE, Banerjee A,Boeree MJ, Salaniponi FM. Treatment outcome of patients with smear-negative and smear-positive pulmonary tuberculosis in the national tuberculosis control programme, Malawi. Trans R Soc Trop Med Hyg. 1999; 93: 443-6.
- Hargreaves NJ, Kadzakumanja O, Whitty CJ, Salaniponi FM, Harries AD, Square SB. Smear-negative pulmonary tuberculosis in a DOTS programme: poor outcomes in an area of high HIV seroprevalence. Int J Tuberc Lung Dis. 2002; 5: 847-54.
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC: Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999; 282: 677-86.
- Bjune G. Tuberculosis in the 21st century: an emerging pandemic? Norsk Epidemiologi. 2005;15(2):133-9.
- Belay M, Bjune G, Ameni G, Abebe F. Diagnostic and treatment delay among tuberculosis patients in Afar region, Ethiopia: a cross-sectional study. BMC Public Health. 2012; 12:369.
- Campos PE, Suarez PG, Sanchez J, Zavala D, Arevalo J, Ticona E, Nolan CM, Hooton TM, Holmes KK. Multidrug-resistant Mycobacterium tuberculosis in HIV-infected persons, Peru. Emerg Infect Dis. 2003; 9: 1571-8.
- Ritacco V, Di Lonardo M, Reniero A, Ambroggi M, Barrera L, Dambrosi A, et al. Nosocomial spread of human immunodeficiency virus-related multidrug-resistant tuberculosis in Buenos Aires. J Infect Dis. 1997; 176: 637-42.
- Moll A, Gandhi N, Pawinski R, et al. Identification of a multi-drug resistant tuberculosis cluster as a cause of death among HIV-co-infected patients in rural South Africa (abstract 795). 13th Conference on Retroviruses and Opportunistic Infections, Denver, CO, USA. 2006; O-115.
- Colebunders R, Bastian I. A review of the diagnosis and treatment of smear-negative pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000; 4: 97-107.
- Perkins MD, Conde MB, Martins M, Kritski AL. Serologic diagnosis of tuberculosis using a simple commercial multiantigen assay. Chest. 2003; 123: 107-12.
- Apers L, Mutsvangwa J, Magwenzi J, Chigara N, Butterworth A, Mason P, et al. A comparison of direct microscopy, the concentration method and the Mycobacteria Growth Indicator Tube for the examination of sputum for acid-fast bacilli. Int J Tuberc Lung Dis. 2003; 7: 376-81.
- Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Britton S, Feleke Y, et al. Evaluation of outpatients with suspected pulmonary tuberculosis in a high HIV prevalence setting in Ethiopia: clinical, diagnostic and epidemiological characteristics. Scandinavian Journal of Infectious Diseases. 2002; 34: 331-7.
- Crampin AC, Floyd S, Mwaungulu F, Black G, Ndhlovu R, Mwaiyeghele E, et al. Comparison of two versus three smears in identifying culture-positive tuberculosis patients in a rural African setting with high HIV prevalence. Int J Tuberc Lung Dis. 2001; 5: 994-9.
- Siddiqi K, Lambert ML, Walley J. Clinical diagnosis of smear-negative pulmonary tuberculosis in low-income countries: the current evidence. Lancet Infect Dis. 2003; 3: 288-96.
- Mefsin MM, Tesfay W, Tasew TW, Richard MJ. The quality of tuberculosis diagnosis in districts of Tigray region of northern Ethiopia. Ethiop J Health Dev. 2005; 19: 13-20.
- Apers L, Wijarajah C, Mutsvangwa J, Chigara N, Mason P, van der Stuyft P. Accuracy of routine diagnosis of pulmonary tuberculosis in an area of high HIV prevalence. Int J Tuberc Lung Dis. 2004; 8: 945–51.
- Post FA, Wood R, Pillay GP. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+ T-lymphocyte count. Tubercle and Lung Dis. 1995; 76: 518-21.
- Greenberg SD, Frager D, Suster B, Walker S, Stavropoulos C, Rothpearl A. Active pulmonary tuberculosis in patients with AIDS: spectrum of radiographic findings (including a normal appearance). Radiology. 1994; 193: 115-9.
- Long R, Maycher B, Scalcini M, Manfreda J. The chest roentgenogram in pulmonary tuberculosis patients seropositive for human immunodeficiency virus type 1. Chest. 1991; 99: 123-7.
- Gupta P, Singh KP, Tripathi AK, Jain A, Prasad R. Role of polymerase chain reaction as diagnostic tool in pulmonary tuberculosis. J Rec Adv Appl Sci. 2013; 28: 19-24.
- Somoskovi A, Hotaling JE, Fitzgerald M. Lessons from a proficiency testing event for acid-fast microscopy. Chest. 2001; 120: 250–7.
- Thakur B, Mehrotra R, Nigam JS. Correlation of various techniques in diagnosis of tuberculous lymphadenitis on fine needle aspiration cytology. Patholog Res Int. 2013; 2013: 824620.
- Jain A, Bhargava A, Agarwal SK. A comparative study of two commonly used staining techniques for acid fast bacilli in clinical specimens. Ind J Tuberculosis. 2002; 49: 161–2.
- Tiruviluamala P, Reichman LB. Tuberculosis. Annu Rev Public Health. 2002; 23: 403–26.
- Zifodya JS, Kreniske JS, Schiller I, Kohli M, Dendukuri N, Schumacher SG, et al. Xpert Ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021; 2: CD009593.
- Gray CM, Katamba A, Narang P, Giraldo J, Zamudio C, Joloba M, et al. Feasibility and Operational Performance of Tuberculosis Detection by Loop-Mediated Isothermal Amplification Platform in Decentralized Settings: Results from a Multicenter Study. J Clin Microbiol. 2016; 54: 1984-91.
- Bjerrum S, Schiller I, Dendukuri N, Kohli M, Nathavitharana RR, Zwerling AA, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev. 2019; 10: CD011420.
- Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet. 2016; 387: 1187-97.
- Lawn SD, Kerkhoff AD, Nicol MP, Meintjes G. Underestimation of the True Specificity of the Urine Lipoarabinomannan Point-of-Care Diagnostic Assay for HIV-Associated Tuberculosis. J Acquir Immune Defic Syndr. 2015; 69: e144-6.
- Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev. 2016; 2016: CD011420.
- Kent TK, Kubica GP. Public Health mycobacteriology. A guide for level III laboratory. Atlanta, Centre for Disease Control. 1985.
- Canetti G, Fox W, Khomenko A, Mahler HT, Menon NK, Mitchison DA, et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull World Health Organ. 1969; 41: 21-43.
- Heifets LB, Cangelosi GA. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int J Tuberc Lung Dis. 1999; 3: 564–81.
- Roberts GD, Goodman NL, Heifets L, Larsh HW, Lindner TH, McClatchy JK, et al. Evaluation of the BACTEC radiometric method for recovery of mycobacteria and drug susceptibility testing of Mycobacterium tuberculosis from acid-fast smear-positive specimens. J Clin Microbiol. 1983; 18: 689-96.
- Siddiqi SH, Libonati JP, Middlebrook G. Evaluation of rapid radiometric method for drug susceptibility testing of Mycobacterium tuberculosis. J Clin Microbiol. 1981; 13: 908–12.
- Palaci M, Ueki SY, Sato DN, Da Silva Tellas MA, Curcio M, et al. Evaluation of mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J Clin Microbiol. 1996; 34: 762-64.
- Sanders CA, Nieda RR, Desmond EP. Validation of the use of Middlebrook 7H10 agar, BACTEC MGIT 960, and BACTEC 460 12B media for testing the susceptibility of Mycobacterium tuberculosis to levofloxacin. J Clin Microbiol. 2004; 42: 5225–8.
- Broccolo F, Scarpellini P, Locatelli G, Zingale A, Brambilla AM, Cichero P, et al. Rapid diagnosis of mycobacterial infections and quantitation of Mycobacterium tuberculosis load by two real-time calibrated PCR assays. J Clin Microbiol. 2003; 41: 4565-72.
- Kataria YP, Khurshid I. Adenosine deaminase in the diagnosis of tuberculous pleural effusion. Chest. 2001; 120: 334–6.
- Larsson L, Mardh PA, Odham G. Detection of tuberculostearic acid in Mycobacteria and Nocardia by gas chromatography & mass spectrophotometry using selected ion monitoring. J. Chromatography. 1979; 163: 221-4.
- French GL, Chan CY, Cheung SW, Oo KT. Diagnosis of acid in sputum by using gas chromatography mass spectrophotometry with selected ion monitoring. J. Infect Dis. 1987; 156: 356-2.
- Mardh PA, Larsson L, Hoiby N, Engback HC, Odham G. Tuberculostearic acid as a diagnostic marker in tuberculous meningitis. Lancet. 1983; 1: 367.