
Research Article
Austin J HIV/AIDS Res. 2016; 3(1): 1020.
The Emerging Histopathologic Diagnostic Challenges of Kaposi Sarcoma in the Acquired Immunodeficiency Syndrome Era
Ramdial PK1*, Miles E1 and Pillay B2
1Department of Anatomical Pathology, National Health Laboratory Service & School of Laboratory Medicine & Medical Sciences, University of KwaZulu Natal, Durban, KwaZulu Natal, South Africa
2Department of Vascular/Endovascular Surgery, Nelson R Mandela School of Medicine, University of KwaZulu Natal, Durban, KwaZulu Natal, South Africa
*Corresponding author: PK Ramdial, Department of Anatomical Pathology, Level 3, Laboratory Building, Inkosi Albert Luthuli Central Hospital, 800 Vusi Mzimela Road, Mayville, 4058, Durban, KwaZulu-Natal, South Africa
Received: January 01, 2016; Accepted: February 11, 2016; Published: February 15, 2016
Abstract
The acquired immunodeficiency syndrome (AIDS) not only catapulted Kaposi Sarcoma (KS) as the commonest AIDS-associated malignancy, but also introduced a wider histomorphological spectrum and an expanded range of diagnostic mimicry and pitfalls. A tissue diagnosis of KS is necessary because of the clinical differential diagnoses, especially in the skin where overlapping features between diseases are visually appreciated. However, the histopathological diagnostic challenges facing the pathologist are not fully recognized. This review highlights the histomorphological challenges that impact the diagnosis of KS in the context of the mimicry of/by traditional KS variants, increased recognition of uncommon variants, emergent histomorphological variants, impact of co-lesional, co-morbid infections and treatment and procedure-related diagnostic challenges.
Keywords: HIV; AIDS; Kaposi’s sarcoma; Pathology; Challenges
Abbreviations
AIDS: Acquired Immunodeficiency Syndrome; AIDS-KS: Acquired Immunodeficiency Syndrome-Associated Kaposi Sarcoma; EBV: Epstein Barr Virus; HAART: Highly Active Anti-Retroviral Therapy; HHV8-LNA-1: Human Herpesvirus 8-Latent Nuclear Antigen-1; HIV: Human Immunodeficiency Virus; KS: Kaposi Sarcoma; LLKS: Lymphangioma-Like Kaposi Sarcoma
Introduction
Kaposi Sarcoma (KS), a multicentric disorder of endothelial origin, is the most frequently observed Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency syndrome (AIDS)- associated malignancy [1]. Although mucocutaneous disease occurs more commonly than visceral disease, the microscopic morphological landscape is characterized by a spindle-shaped cellular neoplastic infiltrate in a richly-vascularized background, a mononuclear cell-rich inflammatory infiltrate, erythrocyte extravasation and edema [2]. The cutaneous spectrum, traditionally encompassing patch, plaque and nodular stages, are well-recognized entities [3]. However, the HIV/AIDS era has introduced an expanded spectrum of histomorphological variants [4,5,6]. Furthermore, the microscopic features of KS lesions maybe impacted by co-morbid, concomitant immunosuppressive diseases [7-13], and Highly Active Anti-Retroviral Therapy (HAART)-induced flares and regression. Finally, challenges arising from secondary AIDS-associated diseases such as lymphedema and biopsy factors may impact the diagnostic recognition of KS [14,15].
This review highlights the histomorphological diagnostic challenges of KS in the HIV/AIDS era in the context of the:
- Mimicry of/by traditional KS variants
- Increased recognition of uncommon variants
- Emergent histomorphological variants
- Impact of co-lesional, co-morbid infections
- Treatment and procedure-related diagnostic challenges
Mimickers of Traditional KS Variants
Although there is a decrease in AIDS-associated KS (AIDS-KS) following HAART implementation in developed countries, this is not the case in developing countries where the incidence of KS continues to increase (3). Apart from the rapid lesional evolution and atypical predilection for the trunk and mucosal surfaces in AIDS patients, visceral involvement also occurs more commonly [16]. The established nodular stage of AIDS-KS does not pose diagnostic dilemmas (Figures 1A-D), but a spindle cell-rich angiosarcoma, pyogenic granuloma and bacillary angiomatosis (Figures 1E-F) may be challenging. While these mimickers lack Human herpes virus 8-latent nuclear antigen-1 (HHV8-LNA-1) immunopositivity, KS lacks the cellular pleomorphism of angiosarcoma, argyrophilic bacillary clusters of bacillary angiomatosis (Figure 1F) and the prominent lobulation and intervascular septa of pyogenic granuloma. The aneurysmal pseudovascular foci, inflammatory component and hemosiderin pigment in aneurysmal fibrous histiocytoma may pose a pitfall but the admixture of foam cells, variable fibrosis and peripheral collagen compartmentalization and HHV8-LNA-1 immunonegativity will enable KS diagnosis [16]. A lobular architecture, intralesional thrombi and absence of eosinophilic globules help differentiate KS from epithelioid hemangioendothelioma [16].
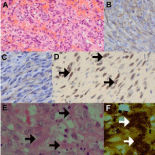
Figure 1: Nodular KS mimicry (original images): Richly vascularized
nodular KS (A, H&E 240X) with brown CD34 (B, 480X) and CD31 (C, 480X)
immunopositivity of spindle cells, confirming an endothelial lineage. HHV8-
LNA-1 brown nuclear immunopositivity (D, arrows, oil immersion 1200X). KS
mimicker, bacillary angiomatosis (E, H&E 480X), composed of richly vascular
background, cellular debris and aggregates of organisms (arrows), highlighted
on Warthin Starry silver stain (F, H&E 240X) as clumped organisms (arrows).
The variable spindle cell infiltrate, inflammatory cells and vasculature of plaque stage AIDS-KS (Figures 2A-B) may mimic granulation tissue, fibrous histiocytoma (Figures 2C-D) and acroangiodermatitis [3,16]. The absence of a back-to-back alignment of the vasculature and HHV8-LNA-1 staining facilitate distinction from KS mimickers. The early patch stage lesion in which there are variable but minor vascular and inflammatory cellular components (Figures 2E-G) shares features with non-specific dermatitis and vascular malformations [3,16,18]. The clinical background and HHV8-LNA-1 immunopositivity of isolated spindle and endothelial cells in hemangioma-like foci underpin diagnostic confirmation of early KS.
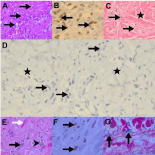
Figure 2: Plaque and patch stage KS mimicry (original images): Plaquestage
Kaposi sarcoma (KS) with slit-like vasculature (A, arrows, H&E 120X)
and HHV8-LNA-1 brown nuclear immunopositivity (B, arrows, oil immersion
1200X) of endothelial cells. KS mimicker, fibrous histiocytoma (C, H&E 240X),
demonstrating dermal fibrosis (asterisk) and vascular channels (arrows) and
inflammatory cells associated with HHV8-LNA-1 immunonegative endothelial
cells (D, arrows, 480X) in a collagenous background (asterisks). Patch stage
KS (E, H&E 240X) with spindled endothelial cells lining vascular slits (black
arrow), erythrocyte extravasation (white arrow) and hemosiderin pigment
(arrowhead) in association with HHV8-LNA-1 nuclear immunopositivity
(F, arrows, oil immersion 1200X) and iron Van Gieson stain (G, 240X)
highlighting hemosiderin deposits (arrows).
Increased Recognition of Uncommon Variants
Anaplastic KS
Anaplastic KS assumes an obvious sarcomatous morphology with cellular pleomorphism, increased mitotic activity and necrosis (Figures 3A-C) [3,4,16,17]. In this context, KS shares features with other malignant soft tissue spindle cell tumors, including those of myoid (Figure 3D), synovial (Figure 3E), endothelial (Figure 3F) and nerve sheath origin [3,4,16,17]. Malignant Epstein Barr Virus (EBV)- induced myoid tumours (Figure 3G) are of particular relevance in AIDS patients because of the association with immunosuppression [19]. The prominent myoid immunoprofile, lack of endothelial CD31 and CD34 and HHV8-LNA-1 immunostaining and presence of an EBV signature (Figure 3H) would support a myoid lesion. The endothelial cells lining vascular channels appear atypical, mimicking angiosarcoma that is HHV-8-LNA-1 immunonegative [20]. While malignant, including desmoplastic, melanoma and nerve sheath tumors may mimic anaplastic KS, the pitfall is enhanced by the recently described presence of S100 protein-positive, bystander, immunosurveillance Langerhans cells in anaplastic KS (Figure 3I) [21].
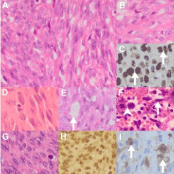
Figure 3: Anaplastic KS mimicry (original images): Anaplastic KS (AKS)
composed of pleomorphic spindle (A, H&E 240X) and round, epithelioid
cells (B, H&E 480X) that are HHV8-LNA-1 immunopositive (C, arrows, oil
immersion 1200X). Architectural mimicry by AKS of leiomyosarcoma (D, H&E
480X) composed of spindle cells with prominent cytoplasmic eosinophilia,
synovial sarcoma (E, H&E 480X) with a pseudoglandular pattern (arrow)
and angiosarcoma (F, H&E 480X) composed of pleomorphic endothelial
cells (arrow) and prominent mitoses (arrowhead). Epstein Barr Virus (EBV)
leiomyosarcoma (G, H&E 480X) composed of spindle cells with nuclear
EBV early RNA in-situ hybridization positivity (H, 240X). S100 protein
immunopositive immunosurveillance cells (I, arrows, 480X).
Lymph edema-associated challenges and variants
Advanced KS is frequently associated with lymphedema [14,15], but whether KS is the cause or effect of lymphedema is conjectural. The exact cause of the lymphedema is also unknown [14]. Lymphatic theories propose lymphatic and venous occlusion or compression, leaky lymphatic channels and altered lymphatic drainage pathways [15]. The inflammatory theories are based on a response to recurrent infections, including repair and organization phenomena encompassing the angiogenetic responses, paracrine fibroblast proliferation and collagen biosynthesis under the influence of inflammatory cytokines and growth factors [14,15]. Lymphedema is characterized by epidermal hyperplasia, hyperkeratosis, papillomatosis and dermal fibrosis, edema, dilated vasculature and perivascular plasma cells and lymphocytes [15]. Early stage KS, with variable inflammatory and angioproliferative features may be masked in a lymphedematous background (Figure 4A) [14]. In the context of chronic lymphedema, fibroma-like nodules and lymphangiectatic, lymphangioma-like and bullous KS morphological variants have gained prominence in the AIDS era.
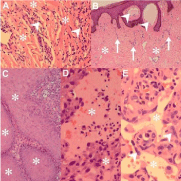
Figure 4: Lymphedema-associated variants and challenges (original
images): Early Kaposi sarcoma (KS) (A, H&E 120X) with slit-like vascular
channels and spindle cells (arrowheads) masked in a collagen-rich
(asterisks), lymphedematous background. Part of a fibroma-like nodule (B,
H&E 120X) demonstrating capillary-caliber vascular channels (arrows) in
a fibrotic background (asterisks) and focal lymphangiectasis (arrowheads).
Pseudoepitheliomatous hyperplasia (C, H&E 240X) with expanded
epidermoid lobules (asterisks). Lymphangiectatic KS (D, H&E 480X)
containing dilated lymphatic channels with pale eosinophilic lymph fluid
(asterisks). Lymphangioma-like KS (E, H&E 480X) containing empty vascular
channels (asterisk) lined by plump spindled endothelial cells (arrowheads).
Fibroma-like nodules
The microscopic features typifying the clinically raised and fleshcoloured fibroma-like nodules include dilated lymphatic and blood vessels, erythrocyte extravasation and hemosiderin amidst looselyarranged collagen bundles and a variable admixture of fibroblasts and fibrosis (Figure 4B) [14,15,22-25]. HHV8-LNA-1 staining is critical to confirm early KS spindle cells in the fibroma-like nodules. Multiple serial sections of the fibroma-like nodules may be necessary to identify focal KS spindle cell infiltrates.
Hyperkeratotic (Verrucous) KS
Documented rarely, this variant is associated with chronic lymphedema, with which it shares the hyperplastic epidermal response (Figure 4C), dermal fibrosis, fibroma-like nodules and lymphangioma-like foci [22,23]. The challenge lies in the confirmation of single or co-lesional disease. Pivotal to the confirmation of subtle KS in the background of chronic lymphedema is the presence of HHV8-LNA-1 spindle cells.
Lymphangiectatic KS
A prominence of dilated intra-and-peritumoral, thin-walled lymphatic channels of varying size and shape in the dermis (Figure 4D) characterize this variant that may be associated with the patch, plaque or nodular stage of KS [4,15]. Superficial aggregation of the lymphangiectatic channels and the accompanying hyperplastic epidermal response mimic lymphangioma circumscriptum that may constitute a pitfall when the typical features of KS are subtle [14].
Lymphangioma-like KS (LLKS)
Described clinically for the first time in 1957 with a ‘bullalike’ [24], compressible morphology, lymphangioma-like KS is characterized microscopically by dermal inter-anastomotic, ectatic, irregularly shaped, empty and variably atypical endothelial-lined spaces (Figure 4E) in a background of otherwise typical KS [22,24]. Slender intraluminal papillae may be present. The vascular dissection is also seen around resident dermal structures and appendages. The dissecting pattern raises potential diagnostic challenges with lymphangioendothelioma, hobnail hemangioma, retiform and spindle cell hemangioendothelioma and low-grade angiosarcoma, none of which are HHV8-LNA-1 immunopositive [24]. The clinical history underpins the distinction of AIDS-KS and lymphangioendothelioma as both may occur in young patients. The latter has a strong association with prior radiotherapy and lacks a spindle cell infiltrate, hemosiderin deposits, erythrocyte extravasation and endothelial atypia. Spindle cell hemangioma shares the presence of cavernous vascular channels and a spindle cell infiltrate with LLKS but it also demonstrates intravascular thrombi and admixed epithelioid cells with intracytoplasmic lumina, and lacks other typical features of KS. Hobnail hemangioma is distinguished from KS by the presence of plump endothelial cells with a banal, epithelioid appearance that protrude into the dissecting vascular spaces [16,18,24]. While lowgrade angiosarcoma stands apart from LLKS because it lacks a spindle cell component, it also demonstrates multilayered endothelial-lined spaces with greater nuclear atypia than LLKS [16,24]. The spindle cell component, lymphocytes and arborizing vasculature of retiform hemangioendothelioma mimic LLKS closely. However, the former contains ‘hobnail’ nuclei and lacks dissection around resident dermal structures, erythrocyte extravasation and hyaline globules [16,24].
Bullous KS
This variant results from prominent superficial dermal peritumoral edema [26]. The bullae maybe intradermally or intraepidermally located.
Newly Recognized Histomorphological Variants
Pyogenic granuloma-like KS
Described as a raised, nodular KS lesion, pyogenic granulomalike KS is characterized by an epidermal collarette, ulceration and a lobulated capillary-caliber vascular proliferation (Figure 5A) [27]. Distinction from KS requires identification of a spindle cell infiltrate (Figure 5B) and additional features of KS, including HHV8-LNA-1 immunopositivity.
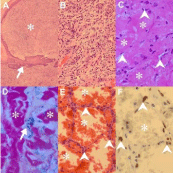
Figure 5: Newly recognized KS variants (original images): Low power view
of pyogenic granuloma-like Kaposi sarcoma (KS) (A, H&E 48X) composed of
a cellular, lobulated, exophytic lesion (asterisk) with an epidermal collarette
(arrow) and higher power demonstration of richly vascularised spindle
cells focus (B, H&E 120X). Keloidal KS (C, H&E 240X; D, iron Van Gieson
240X) with glassy collagen fibres (C & D, asterisks) and intervening slit-like
capillaries (C, arrowheads) and hemosiderin (D, arrow). Telangiectatic KS (E,
H&E 240X; F: HHV8-LNA-1 240X) composed of ectatic, congested vascular
channels (asterisks E & F) lined by spindled endothelial cells (E, arrowheads)
that are HHV8-LNA-1 immunopositive (F, arrowheads).
Keloidal KS
Keloidal KS refers to the presence of dense dermal sclerosis with a glassy appearance of the collagen (Figure 5C) [28]. There may be accompanying hemosiderin (Figure 5D), scar tissue and established KS
Telangiectatic KS
Variably sized, ectatic, congested vascular channels (Figure 5E) lined by HHV8-LNA-1 positive endothelial cells (Figure 5F), amidst foci of typical nodular KS characterize this variant [29] that may be misdiagnosed as an aneurysmal fibrous histiocytoma or sinusoidal hemangioma, because of the dilated ectatic vascular channels. Only KS, however, has HHV8-LNA-1 lined endothelial cells.
Ecchymotic KS
Ecchymotic KS comprises scattered HHV8-LNA-1 spindle cells that are overrun by extravasated erythrocytes [4,30]. Clinically and microscopically, the KS has the appearance of a bruise. Recognition of the masked HHV8-LNA-1 spindle cells undergirds the recognition of KS.
Intravascular KS
Typified by intravenous proliferation of nodular KS in whorls and short bundles of spindle cells with intervening slit-like vascular channels, intravascular KS may be exclusively or focally intravascular [31]. Whether intravascular growth represents extension of extra vascular KS to an intravascular location or whether it is a primary intravascular proliferation is conjectural. The intravascular location of KS may mimic intravascular papillary endothelial hyperplasia, myopericytoma, pyogenic granuloma, and papillary intralymphatic angioendothelioma (Dabska tumor), none of which will be HHV8- LNA-1 immunopositive. Myopericytoma also contains a perivascular, variably whorled and spindled cellular proliferation of myopericytic rather than of endothelial origin [31]. Papillary endothelial hyperplasia is composed of reactive papillary structures containing attenuated endothelial cells without atypia. Dabska tumor is composed of dilated vascular channels with prominent intraluminal endothelial papillary tufts [31]. The latter contain a central hyaline core and surrounding lymphocytes.
Other uncommon variants
KS with myoid nodules [5] is characterized by nodular KS and intratumoral actin-positive, endothelial and HHV8-LNA-1 immunonegative myoid nodules (Figures 6A-C). Whilst the spindle cell component has compelling features of KS, there may be similarities with similar myoid nodules in dermatofibrosarcoma protuberans that also contains CD34-positive spindle cells but does not share the CD31 endothelial and HHV8 infective profile. EBV studies are essential in this context to exclude concomitant EBV-associated myoid proliferations. Glomeruloid KS, typified by glomeruloid, tufted vascular proliferations in an otherwise typical plaque stage KS, may mimic glomeruloid hemangioma, acquired tufted hemangioma and Kaposi form hemangioendothelioma, none of which share HHV8-LNA-1 immunopositivity [5]. While hemosiderin pigment may contribute partially to the dark blue-black profile of KS, melanin pigment in S100 protein-positive Langerhans or dendritic cells must not be confused with pseudovascularized melanocytic lesions [5] (Figure 6D). Micronodular KS is an unencapsulated but circumscribed, small HHV8-LNA-1 positive spindle dermal proliferation [32].
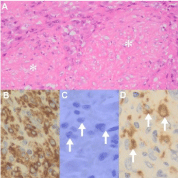
Figure 6: Additional uncommon KS variants (original images): Kaposi
sarcoma with myoid nodules (A, asterisks, H&E 240X) composed of plump
cells that are a-smooth muscle actin positive (B, 480X) and HHV8-LNA-1
immunonegative (C, arrows, oil immersion 1200X). S100 protein positive
pigmented cells (D, arrows, 480X).
KS Impacted by Co-Morbid Infections
AIDS-associated cell-mediated dysfunction predisposes patients to a spectrum of AIDS-defining, infections and neoplasms [33]. The dual identification of infections and neoplasms in a single patient is reported uncommonly [9,12,34-36]. The co-lesional occurrence of KS and co-morbid infections is a rarely documented phenomenon [11,12]; to date a range of fungal, bacterial and parasitic co-infections have been reported co-lesionally with KS (Table 1) [12]. The challenges associated with the co-lesional occurrence of KS and granulomas are multi-pronged because of the wide microscopic differential diagnosis, in general, and in the context of AIDS immunosuppression and immune restoration, specifically (Table 2). Mycobacterial infections [12,36] may present with necrotizing and non-necrotizing granulomatous (Figures 7A-C) and as pseudotumoral (Figures 7D-E ) responses [36,37]. The necrosis may be caseative, karyorrhectic or suppurative in nature and the non-necrotizing granulomas may be of sarcoidal character. The role of special and ancillary tests in determining the cause of the granulomatous inflammation cannot be undermined [12,37]. Careful appraisal of Ziehl-Neelsen, Fite- Faraco, periodic acid Schiff, mucicarmine and silver stains (Gomori Grocott methenamine silver and methenamine silver) underpin the morphological identification of organisms causing granulomatous responses. However, the absolute identification of mycobacterial subtype requires molecular or microbiological confirmation.
A. Mimickers of/by traditional KS variants
1. Patch stage:
- Dermatitis
- Hemangioma
- Stasis dermatitis
2. Plaque stage:
- Acroangiodermatitis
3. Nodular stage:
- Infective: bacillary angiomatosis
- Neoplastic (benign): fibrous histiocytoma
- Neoplastic (malignant): sarcoma, carcinoma
B. Challenges of uncommon variants
1. Anaplastic KS mimicry:
- Angiosarcoma
- Malignant myoid tumors
- Synovial sarcoma
- Nerve sheath malignancy
2. Lymphedema-associated challenges and variants
- Confirmation of lymphedema
- Fibroma-like nodules
- Hyperkeratotic (verrucous) KS
- Lymphangiectatic KS
- Lymphangioma-like KS
- Bullous KS
C.Newly recognized variants
- Pyogenic granuloma-like KS
- Keloidal KS
- Telangiectatic KS
- Ecchymotic KS
- Intravascular KS
- Other: micronodular, glomeruloid
D. KS impacted by co-morbid infections
1. Mycobacterial granulomatous inflammation
- Necrotizing, non-necrotizing, pseudotumoral
2. Cryptococosis
3. Cytomegalovirus
4. Herpes simplex virus
5. Molluscum contagiosum virus
E. KS impacted by clinical treatment/procedure
1. Impact of anti-retroviral therapy
- KS regression
- KS flare
2. Impact of biopsy factors
- Superficial nature
- Traction artefact
- Ulceration
- Processing issues
- HHV8-LNA-1 staining
Table 1: Cutaneous Histopathological Diagnostic Challenges of Kaposi Sarcoma (KS).
A. Infection-related
1. Mycobacterium tuberculosis
2.Mycobacterium other than tuberculosis
3. Cryptococcus neoformans
4. Histoplasmosis capsulatum
5. Others
B. Immune host response to:
1. Tumor antigens
2. Tumor directly
C. Drug-related
1. Unrelated to KS therapy
- Interstitial granulomatous dermatitis
- Granuloma annulare-like
2. Related to KS therapy
- Interferon-a
- Chemotherapy
3. Immune reconstitution
- Sarcoidosis
- Foreign material
Table 2: Etiology of Kaposi sarcoma (KS)-related and acquired immunodeficiency syndrome-associated granulomas.
Infective pseudotumoral responses may pose diagnostic challenges and mimicry of spindle cell tumors [36]. Although the pseudotumoral response and KS share a spindle cell infiltrate (Figure 7D), the former has a dominant histiocytic or combined histiocytic / myofibroblastic immunophenotype [13]. Amongst mycobacterial infections, Mycobacterium other than tuberculosis produces pseudotumoral responses more commonly than M. tuberculosis [36]. However infective spindle cell pseudotumoral responses are also caused by C.neoformans [13] and H. capsulatum [38]. A high index of suspicion for infections is necessary when appraising spindle cell lesions in patients within the infiltrate or when the organisms are not identifiable on routine hematoxylin and eosin-stained sections. The spindle cells of Kaposi sarcoma are HHV8-LNA-1 immunopositive whereas the pseudotumoral response will demonstrate a histiocytic and/or myofibroblastic immunophenotype.
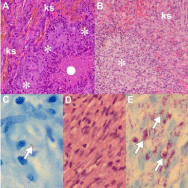
Figure 7: KS impacted by co-morbid Mycobacterial infections (original
images): KSat periphery (A & B, ks, H&E 240X) with necrotizing (A, white solid
circle) granulomatous inflammation (A, asterisks), non-necrotising granuloma
(B, asterisk) and acid fast bacillus on Ziehl-Neelsen stained section (C, arrow,
oil immersion 1200X). Mycobacterial spindle cell pseudotumor (D, H&E 480X)
with myriads acid fast bacilli (E, arrows, Ziehl Neelsen oil immersion 1200X).
Intratumoral non-necrotizing, tumor-related, sarcoidal granulomas (Figure 7B) may be a consequence of infections caused by depressed immunity, an immune host reaction to tumor substances or neoplastic cells, or a drug-induced cause [39-41]. Tumor-like antigens are implicated in granulomatous inflammation in epidemic KS in which all infective causes of granulomatous inflammation are excluded [41]. Immune reconstitution syndrome may also be responsible for necrobiotic and foreign body granulomas [12]. Drug- induced interstitial granulomatous dermatitis may be caused by a range of drugs used in the treatment of AIDS patients. Interferon-a directly inhibits KS cell proliferation and modulation of host immune responses by an anti-neoplastic effect [39,40]. In contrast to the dual mycobacterial pathology that challenges KS diagnosis because the organisms are not seen on routine stains, the diagnosis of fungal, viral and parasitic infections rests on the identification of well-recognized histomorphological features of the infective agents. These may be altered, however, because of prior treatment or may be too sparse to be identified on routine stains. C. neoformans is easily recognized through its soap-bubble appearance and prominent mucinous wall (Figure 8A-B) but capsule-deficient forms in immunosuppressed patients have thin capsules [13]. Similarly, histoplasmosis may be poorly detected in KS [37] because of its small size or densely inflamed background of immune reconstitution. Although the exact pathogenesis of co-lesional cryptococcosis and KS remains unconfirmed, possible pathogenetic mechanisms include inflammatory oncotaxis, koebnerization and immune reconstitution [8,11,42]. Inflammatory oncotaxis, the attraction of cancer cells and its transcapillary migration into tissue spaces, remote to the primary tumor site, has been proposed when the cryptococcal infection effects local oncotaxis adequate enough to promote KS at the infective site [11]. The proposed pathomechanism for koebnerization encompasses the release of basic fibroblast growth factor at sites of prior trauma by traumatized keratinocytes [11]. KS not only provides an optimal environment for cryptococcal colonization during episodes of cryptococcemia but also protects intralesional organisms from antifungal therapy [42].
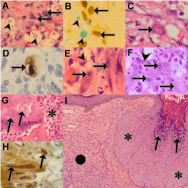
Figure 8: KS impacted by co-morbid fungal and viral infections (original
images): KS (A, H&E 480X) with spindle cells (arrows) and co-lesional
cryptococcal yeast forms (arrowheads). Dual stain (480X) demonstrating
HHV8-LNA-1 immunopositive nuclei (B, arrows) with alcian green counterstain
highlighting C.neoformans mucinous capsules (arrowheads). Focal large
cytomegalovirus ‘Owl’s eye’ intranuclear inclusion (C, arrow, H&E oil immersion
1200X) in KS confirmed with cytomegalovirus immunohistochemical stain
(D, arrow, oil immersion 1200X). Atypical spindled (E, arrows, oil immersion
1200X) and plump (F, arrows, H&E oil immersion 1200X) cytomegalovirus
inclusions in angiomatous KS background (E, F: arrowheads). Ulcerated KS
(G, H&E 480X) with inflammatory exudate (asterisk) and intranuclear ground
glass herpetic inclusions (arrows), immunopositive with herpes simplex virus
immunohistochemistry (H, arrows, oil immersion 1200X). KS (I, H&E 120X)
with a nodular appearance (solid circle) and molluscum contagiosum virus
infection (asterisk) with myriads molluscum bodies (arrows)..
Viral infections, including cytomegalovirus, herpes simplex and molluscum contagiosum virus, are identified by their inclusion bodies (Figures 8C-I). Atypical, including spindled forms of cytomegalovirus may be overlooked as plump fibroblasts (Figure 8C). Heightened awareness is necessary for microscopic identification. While cytomegalovirus has been assigned an etiopathogenetic role in KS, in conjunction with HHV8, herpes simplex virus and human herpesvirus 6, through cytokine activation and endothelial proliferation [10,43], its exact colonizing or causative role remains controversial. Herpes simplex virus inclusions may be noted at the edge of ulcerated epidermis or mucosal squamous epithelium (Figure 8G) [27]. Regenerate epidermal features may mimic nuclear herpetic inclusions and recourse to immunohistochemistry for viral confirmation (Figure 8H) may be necessary. Syncytial herpetic giant cells may also be a valuable aid to diagnosis in this context.
Procedure and Treatment-Related Challenges
Impact of highly active anti-retroviral therapy
KS regression: KS regression may be induced by several iatrogenic means. Spontaneous regression of KS occurs in 2-10% of classic KS while transplant-associated KS may regress following immunosuppressant withdrawal or dose decrease [6]. AIDS-associated KS may regress partially or completely following chemotherapy and/ or HAART [6,43]. Partially regressed lesions demonstrate fibrosis (Figure 9A), prominence of the dermal vasculature (Figures 9A-B) and a decrease in the spindle cell component of the neoplastic lesions while complete regression is characterized by an absence of spindle cells (Figure 9A). The confirmation of KS in regressing lesions is further challenged by a decrease or absence of HHV8-LNA-1 staining of the lesional foci (Figure 9C). Additional features of regression include extensive sclerosis (Figure 9D), a superficial perivascular lymphocytic infiltrate and abundant dermocentric hemosiderinladen macrophages (Figure 9E). Biopsies of KS lesions that have been subjected to intralesional therapy [44] demonstrate dense dermal sclerosis.
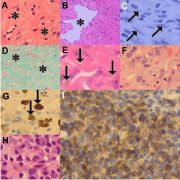
Figure 9: Impact of highly active anti-retroviral therapy on KS morphology
(original images): Regressing KS (A, H&E 240X) characterized by dermal
scarring (asterisks), reduction of spindle cells and capillary dilatation, lymphatic
dilatation (B, asterisk, H&E 240X) and variable, including negative, HHV8-
LNA-1 immunostaining (C, arrows, oil immersion 1200X). Masson trichrome
stain highlights dermal fibrosis (D, asterisks, 120X). Hemosiderin deposition
(E, arrows, H&E 480X). KS flare characterized by plump endothelial cells (F,
H&E 480X) with irregular nuclear contours that are accentuated by HHV8-
LNA-1 immunostaining (G, arrows, oil immersion 1200X). KS flare containing
a dense lymphocytic inflammatory infiltrate (H, H&E 480X) of dominant CD3/
CD8-positive immunophenotype (I, CD8 oil immersion 1200X).
KS Flare: While the use of HAART has decreased the prevalence of AIDS-KS, a paradoxical worsening of KS may occur in HIV-infected patients who are undergoing immune system recovery [45,46]. Variably referred to as KS flare, recrudescence or exacerbation [47], with variably increased vascularity or inflammation (Figures 9F-I), it may also be associated with corticosteroid and rituximab therapy [48]. While the histomorphology of KS flare as part of immune reconstitution is similar to that in nodular KS, a change from a multi nodular lesion with a poorly circumscribed border to a uninodular, well-circumscribed lesion that is less cellular and surrounded by a dense fibrotic stroma, is also documented [49]. Rituximab-induced KS recrudescence is associated with an absence of B-lymphocytes [48].
Impact of biopsy factors: Clinical and laboratory-based
Superficial nature: Because of the vascular nature of KS many biopsies are superficial. As a result the biopsy may include either hyperplastic and hyperkeratotic epidermis from lymphedematous extremities or ulcerated epidermis and superficial acute inflammatory exudate. The dermis may be unrepresented or superficially represented (Figure 10A). In such settings, HHV8-LNA-1 spindle cell immunopositivity will attest to the presence of KS but for optimal morphological evaluation, a deeper biopsy from the same location can be confidently advised.
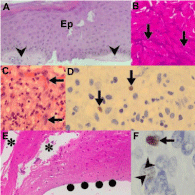
Figure 10: Impact of biopsy factors on KS diagnosis (original images):
Superficial biopsy (A, H&E 240X) from a lymphedematous extremity with
acanthotic epidermis (Ep) and minimal dermal representation (arrowheads).
Surgery-induced traction and crush artifact (B, H&E 240X) precluding
identification of morphological detail (arrows). Biopsy of ulcerated lesion (C,
H&E 240X) with exudate and focal spindle cells (arrows) that are HHV8-
LNA-1 immunopositive (D, arrows, oil immersion 1200X). Poor adhesion of
tissue sections (E, H&E 240X) causes fallout of tissue sections (asterisks)
and dehiscence of the epidermis at the dermo-epidermal junction (solid
circles). Variable HHV8-LNA-1 immunostaining (F, oil immersion 1200X) with
bright, dense (arrow) and pale, dot-like (arrowheads) quality.
Traction artifact: Because of the firm consistency of lymphedematous tissue, biopsies may be distorted by traction and crush artifacts that preclude appraisal of the spindle cell nature of the lesion (Figure 10B). This may be a consequence of clinical handling of the tissue or may occur in the laboratory, during specimen put-through when resistance to gross sectioning of the tissue is experienced. Traction-distorted tissue sections appear hyper chromatic and cellular. A temptation to confirm malignancy should not be raised if the morphological features of KS are not identified.
Ulceration: Ulcerated tissue is typified by an inflammatory granulation tissue and exudative inflammatory response (Figure 10C). HHV8-LNA-1 testing is mandatory to confirm immunopositive neoplastic cells either admixed with, or masquerading as, myofibroblasts (Figure 10D).
Processing issues: Poor glass slide tissue adhesion: Biopsies with hyperkeratotic and hyperplastic epidermis may demonstrate poor adherence to glass slides (Figure 10E). Section pick up on charged slides may facilitate optimal adherence of sections.
HHV8-LNA-1 staining: Not only did the discovery of HHV8 as the causative agent of KS signify a landmark in the history of the malignancy, but the commercial availability of the antibody, HHV8-LNA-1, has enabled diagnostic confirmation in suboptimal, challenging situations [50,51]. However, HHV8-LNA-1 immunostaining is variable (Figure 10F) and may be restricted to a dot-like nuclear pattern, especially following antiretroviral therapy. Hence, assessment of seemingly immunonegative stains in a compelling clinical or histomorphological setting mandates close high power observations of the stained sections. The HAART-induced immunonegativity may mandate additional deeper sectioning in pursuit of intralesional tumor heterogeneity or multiple additional biopsies of nodular KS clinical lesions.
Conclusion
The increased exposure of histopathologists to biopsies of KS, a consequence of the increased incidence of KS in the AIDS era, has resulted in the recognition of an expanding spectrum of histomorphological variants. In addition, the AIDS immunosuppression has resulted in co-morbid co-lesional pathology of a range of infective entities, some identifiable by histomorphological traits and others by histomorphological reaction patterns. The histopathological appraisal of KS in the AIDS era therefore requires careful scrutiny to not only confirm the diagnosis of KS, but also to identify unrecognized concomitant, co-lesional disease that may represent the sentinel of eminently treatable systemic disease.
Acknowledgement
We thank Mrs M Moodley for her assistance in manuscript typing and formating, and literature retrieval and Mr Dinesh Sookhdeo for assistance with laboratory work and photography.
References
- Letang E, Lewis JJ, Bower M, Mosam A, Borok M, Campbell TB, Naniche D. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma: higher incidence and mortality in Africa than in the UK. AIDS. 2013; 27: 1603-1613.
- Pantanowitz L, Khammissa RA, Lemmer J, Feller L. Oral HIV-associated Kaposi sarcoma. J Oral Pathol Med. 2013; 42: 201-207.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013; 137: 289-294.
- Grayson W1, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008; 3: 31.
- O'Donnell PJ, Pantanowitz L, Grayson W. Unique histologic variants of cutaneous Kaposi sarcoma. Am J Dermatopathol. 2010; 32: 244-250.
- Pantanowitz L, Dezube BJ, Pinkus GS, Tahan SR. Histological characterization of regression in acquired immunodeficiency syndrome-related Kaposi’s sarcoma. J Cutan Pathol. 2004; 31: 26-34.
- Prasad Busarla SV, Sayed S, Nazarian RM, Gimbel DC, Moloo Z, Sohani AR. Kaposi sarcoma in association with molluscum contagiosum: an uncommon diagnosis in a single biopsy and potential diagnostic pitfall. Am J Dermatopathol. 2012; 34: e7-9.
- Glassman SJ, Hale MJ. Cutaneous cryptococcosis and Kaposi's sarcoma occurring in the same lesions in a patient with the acquired immunodeficiency syndrome. Clin Exp Dermatol. 1995; 20: 480-486.
- Lanjewar D. Co-existent lymphoma and tuberculosis and Kaposi’s sarcoma with tuberculosis occurring in lymph node in patient with AIDS: Report of two cases. Histopathology. 2008; 53: 6-7.
- Meer S, Altini M. Cytomegalovirus co-infection in AIDS-associated oral Kaposi's sarcoma. Adv Dent Res. 2006; 19: 96-98.
- Ramdial PK, Sing Y, Subrayan S, Calonje E. cutaneous colesional acquired immunodeficiency syndrome associated Kaposi sarcoma and cryptococcosis. Am J Dermatopathol. 2010; 32: 780-786.
- Ramdial PK, Sing Y, Subrayan S, Calonje E, Aboobaker J, Sydney C, et al. Granulomas in acquired immunodeficiency syndrome-associated cutaneous Kaposi sarcoma: evidence for a role for Mycobacterium tuberculosis. J Cutan Pathol. 2010; 37: 827-834.
- Sing Y, Ramdial PK. Cryptococcal inflammatory pseudotumors. Am J Surg Pathol. 2007; 31: 1521-1527.
- Ramdial PK, Chetty R, Singh B, Singh R, Aboobaker J. Lymphedematous HIV-associated Kaposi's sarcoma. J Cutan Pathol. 2006; 33: 474-481.
- Pantanowitz L, Duke WH. Lymphoedematous variants of Kaposi's sarcoma. J Eur Acad Dermatol Venereol. 2008; 22: 118-120.
- Calonje E, Brenn T. Vascular tumors: Tumors and tumorlike conditions of blood vessels and lymphatics. Elenitsas R, Rosenbach M, Murphy GF, Rubin AI, Xu X, editors. In: Lever’s Histopathology of the Skin. Wolters Kluwer. 2015; 1251-1310.
- Satta R, Cossu S, Massarelli G, Cottoni F. Anaplastic transformation of classic Kaposi's sarcoma: clinicopathological study of five cases. Br J Dermatol. 2001; 145: 847-849.
- Goldblum JR, Folpe AL, Weiss SW. Malignant Vascular Tumors. Goldblum JR, Folpe AL, Weiss SW, editors. In: Enzinger & Weiss’s Soft Tissue Tumors. Elsevier Saunders, Philadelphia; 2014: 703-732.
- Ramdial PK, Sing Y, Deonarain J, Vaubell JI, Naicker S, Sydney S, et al. Extra-uterine myoid tumours in patients with acquired immunodeficiency syndrome: a clinicopathological reappraisal. Histopathology. 2011; 59: 1122-1134.
- Craddock KJ, Labonte S, Ghazarian D. Anaplastic Kaposi sarcoma resembling epithelioid angiosarcoma in an HIV-positive man. Eur J Dermatol. 2008; 18: 358-359.
- Ramdial PK, Sing Y, Naicker S, Calonje E, Sewram V, Singh B. Langerhans cells in anaplastic Kaposi sarcoma with a paucivascular phenotype: a potential diagnostic pitfall. Pathol Int. 2011; 61: 221-227.
- Cossu S, Satta R, Cottoni F, Massarelli G. Lymphangioma-like variant of Kaposi's sarcoma: clinicopathologic study of seven cases with review of the literature. Am J Dermatopathol. 1997; 19: 16-22.
- Davis DA, Scott DM. Lymphangioma-like Kaposi's sarcoma: etiology and literature review. J Am Acad Dermatol. 2000; 43: 123-127.
- Ramirez JA, Laskin WB, Guitart J. Lymphangioma-like Kaposi sarcoma. J Cutan Pathol. 2005; 32: 286-292.
- Caputo R, Gianotti R, Grimalt R, Monti M, Alessi E. Soft fibroma-like lesions on the legs of a patient with Kaposi's sarcoma and lymphedema. Am J Dermatopathol. 1991; 13: 493-496.
- Recht B, Nickoloff BJ, Wood GS. A bullous variant of Kaposi's sarcoma in an elderly female. J Dermatol Surg Oncol. 1986; 12: 1192-1197.
- Scott PL, Motaparthi K, Krishnan B, Hsu S. Pyogenic granuloma-like Kaposi sarcoma: a diagnostic pitfall. Dermatol Online J. 2012; 18: 4.
- Schwartz RA, Spicer MS, Janniger CK, Cohen PJ, Melczer MM, Lambert WC. Keloidal Kaposi's sarcoma: report of three patients. Dermatology. 1994; 189: 271-274.
- Snyder RA, Schwartz RA. Telangiectatic Kaposi's sarcoma. Occurrence in a patient with thymoma and myasthenia gravis receiving long-term immunosuppressive therapy. Arch Dermatol. 1982; 118: 1020-1021.
- Schwartz RA, Spicer MS, Thomas I, Janniger CK, Lambert WC. Ecchymotic Kaposi's sarcoma. Cutis. 1995; 56: 104-106.
- Luzar B, Antony F, Ramdial PK, Calonje E. Intravascular Kaposi's sarcoma - a hitherto unrecognized phenomenon. J Cutan Pathol. 2007; 34: 861-864.
- Kempf W, Cathomas G, Burg G, Trüeb RM. Micronodular Kaposi's sarcoma - a new variant of classic-sporadic Kaposi's sarcoma. Dermatology. 2004; 208: 255-258.
- Cole MC, Cohen PR, Satra KH, Grossman ME. The concurrent presence of systemic disease pathogens and cutaneous Kaposi's sarcoma in the same lesion: Histoplasma capsulatum and Kaposi's sarcoma coexisting in a single skin lesion in a patient with AIDS. J Am Acad Dermatol. 1992; 26: 285-287.
- Croxson TS, Ebanks D, Mildvan D. Atypical mycobacteria and Kaposi's sarcoma in the same biopsy specimens. N Engl J Med. 1983; 308: 1476.
- Kaminski Z. Various opportunistic infections and neoplasms in patients dying of AIDS in the last 12 years – report based on pathomorphological investigations. Med Sci Monit. 2001; 7: 421-426.
- Logani S, Lucas DR, Cheng JD, Loachim HL, Adsay NV. Spindle Cell Tumors Associated with Mycobacteria in Lymph Nodes of HIV-Positive Patients: ‘Kaposi Sarcoma with Mycobacteria’ and ‘Mycobacterial Pseudotumor’. Am J Surg Pathol. 1999; 23: 656-661.
- Ramdial PK. Dermatopathological challenges in the human immunodeficiency virus and acquired immunodeficiency syndrome era. Histopathology. 2010; 56: 39-56.
- Den Bakker MA, Goemaere NNT, Severin JA, Nouwen JL, Verhagen PCMS. Histoplasma-associated Inflammatory Pseudotumour of the Kidney Mimicking Renal Carcinoma. Virchows Arch. 2009; 454: 229-232.
- Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986; 13: 147-156.
- Brunner A, Kantner J, Tzankov A. Granulomatous reactions cause symptoms or clinically imitate treatment resistance in small lymphocytic lymphoma/chronic lymphocytic leukaemia more frequently than in other non-Hodgkin lymphomas. J Clin Pathol. 2005; 58: 815-819.
- Kandemir NO, Yurdakan G, Bektas S, Tekin NS. Classic Kaposi sarcoma with sarcoid-like granulomas: a case report and literature review. Exp Mol Pathol. 2009; 87: 89-93.
- Sofman MS, Heilman ER. Simultaneous occurrence of Kaposi's sarcoma and Cryptococcus within a cutaneous lesion in a patient with acquired immunodeficiency syndrome. Arch Dermatol. 1990; 126: 683-684.
- Cattelan AM, Calabro ML, Gasperini P, Aversa SML, Zanchetta M, Meneghetti F, et al. Acquired Immunodeficiency Syndrome-Related Kaposi’s Sarcoma Regression after Highly Active Antiretroviral Therapy: Biologic Correlates of Clinical Outcome. J Natl Cancer Inst Monogr. 2001; 28: 44-49.
- Burdick AE, Carmichael C, Rady PL, Tyring SK, Badiavas E. Resolution of Kaposi's sarcoma associated with undetectable level of human herpesvirus 8 DNA in a patient with AIDS after protease inhibitor therapy. J Am Acad Dermatol. 1997; 37: 648-649.
- Papagatsia Z, Jones J, Morgan P, Tappuni AR. Oral Kaposi sarcoma: a case of immune reconstitution inflammatory syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 108: 70-75.
- Naidu TK, Ramdial PK, Naidoo SK. Highly active antiretroviral therapy-associated flare of oropharyngeal Kaposi sarcoma. Arch Otolaryngol Head Neck Surg. 2012; 138: 762-764.
- Leidner RS, Aboulafia DM. Recrudescent Kaposi's sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS. 2005; 19: 635-644.
- Pantanowitz L, Früh K, Marconi S, Moses AV, Dezube BJ. Pathology of rituximab-induced Kaposi sarcoma flare. BMC Clin Pathol. 2008; 8: 7.
- Eng W, Cockerell CJ. Histological features of kaposi sarcoma in a patient receiving highly active antiviral therapy. Am J Dermatopathol. 2004; 26: 127-132.
- Cheuk W, Wong KO, Wong CS, Dinkel JE, Ben-Dor D, Chan JK. Immunostaining for human herpesvirus 8 latent nuclear antigen-1 helps distinguish Kaposi sarcoma from its mimickers. Am J Clin Pathol. 2004; 121: 335-342.
- Nuovo M, Nuovo G. Utility of HHV8 RNA detection for differentiating Kaposi's sarcoma from its mimics. J Cutan Pathol. 2001; 28: 248-255.