
Review Article
Austin J HIV/AIDS Res. 2016; 3(2): 1026.
Anti-Retroviral Drugs for HIV: Old and New
Vaidya KA, Kadam AV and Nema V*
National AIDS Research Institute, 73 G Block, M.I.D.C Bhosari, India
*Corresponding author: Vijay Nema, National AIDS Research Institute, 73 G Block, M.I.D.C Bhosari, India
Received: April 25, 2016; Accepted: June 06, 2016; Published: June 08, 2016
Abstract
Treatment of HIV started as monotherapy initially, and then multiple drugs in regimens were given where patients had to consume 11-16 tablets per day. Now the mainstay of the treatment is a single fixed dose combination of Tenofovir, Lamivudine and Efavirenz per day or Zidovudine, Lamivudine and Nevirapine twice daily. Toxicity, resistance and adherence still remaina crucial issue. We need long acting depot preparations which would be efficacious for prevention, treatment and have fewer side effects. To implement test and treat policy promoted by WHO, regular supply of cost effective antiretroviral drugs and newer drugs which would get approved remains a challenge for developing countries. Hence we tried reviewing upcoming new molecules which showed potential to be good drugs in various phases of clinical trial.
Keywords: Newer Anti retroviral drugs; HIV; US FDA approved ARV drugs
Introduction
HIV (Human Immunodeficiency Virus) is a retrovirus that gradually attacks the immune system, which protects human body against illness. HIV infected person becomes a easy target for opportunistic infections and diseases. This virus multiplies in T-helper cell (CD4) and gradually depletes them. The two main types are HIV-1 and HIV-2. HIV-1 is the most common type found worldwide; However HIV-2 is found mainly in Western Africa, with some cases in India and Europe [1]. Currently, around 15 molecules are being used in different treatments regimens to treat HIV infections, however, issues like treatment failure due to drug resistance and toxicity remain crucial issues. The purpose of this review is to brief about newer antiretroviral drugs (Pharmacokinetics and pharmacodynamics) for HIV, which are recently approved and/ or newer promising drugs in pipeline i.e. phase 2 and phase 3 trials. Using newer antiretroviral drugs as keyword in PubMed, DAIDS, CDC website, AIDS info FDA websites were searched thoroughly for recent updates.
Basic Facts about HIV
HIV infected person would develop AIDS in 10 to 15 years which is last clinical stage. HIV mainly found in blood, semen, vaginal and anal fluids and breast milk. However, it cannot be transmitted through sweat, saliva or urine. Currently, there is no cure for HIV but with early diagnosis and effective Antiretroviral (ARV) treatment, people with HIV can live a long and normal, healthy life. Therefore, it is important to take correct treatment regularly. The drugs currently available for HIV blocks the replication by interfering at various stages of the life cycle. These drugs have their own toxicities and many have reported development of resistance. We have left with very few options in our arm our against HIV.
HIV Virus Life Cycle and Antiviral Drug Targets (Figure 1)
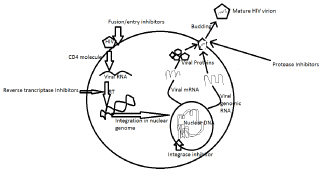
Figure 1: HIV virus life cycle and antiviral drug targets.
Classification of antiretroviral (ARV) drugs [2]
The table below shows the classification of antiretroviral drugs.
We have further divided each class depending on the FDA approval of the drugs before and after 2012. It is quite evident that number of drugs approved after 2012 has dropped down. This may be due to decreased prevalence and increased cost of clinical trials (Table 1, Figure 2).
Nucleoside reverse transcriptase inhibitor (NRTI)
Non-Nucleoside reverse transcriptase inhibitor (NNRTI)
Protease Inhibitor(PI)
Fusion inhibitor
Entry inhibitor
Integrase inhibitor
ARV drugs approved by FDA before 2012
Zidovudine
Delavirdine
Atazanavir
Enfuvirtide
(T-20)
Maraviroc
(MVC)
Raltegravir
Stavudine
Efavirenz
Darunavir
Lamivudine
Etravirine
Fosamprenavir
Emtricitabine
Rilpavirine
Indinavir
Abacavir
Nevirapine
Nelfinavir
Didanosine
Ritonavir
Zalcitabine
Saquinavir
Tenofovir
Tipranavir
Amprenavir
Lopinavir
ARV drugs approved by FDA after 2012
Tenofovir Alafenamide
Dolutegravir
Elvitegravir
Cabotegravir
*Cobicistat acts as Pharmacokinetic Enhancers (CYP3A Inhibitors) which do not have any antiviral activity.
Table 1: Classification of antiretroviral (ARV) drugs.
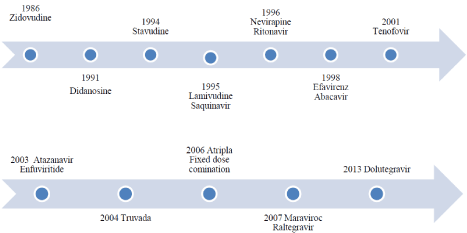
Figure 2: Timeline for ARV development [3].
In patients experiencing virological failure, assessment of adherence to treatment is helpful to determine the mechanisms of failure and to choose an alternative therapeutic option [4]. Treatment of HIV with medicines is called as Highly Active Anti- Retroviral Therapy (HAART). ART is recommended for everyone who are HIV positive as per new who guidelines. Various clinical trials have determined the combinations that are optimal to use as first-line therapy. Rigorous investigations are needed to establish the value of simplified regimens in an attempt to increase the adherence. People on ART take a combination of HIV medicines every day. A person’s initial HIV regimen generally includes three HIV medicines from at least two different drug classes. ARV drug treatment cannot cure HIV, but help people with HIV live longer and healthier lives. ARV medicines also reduce the risk of HIV transmission.
Guideline for initiation of anti retroviral drugs [5]
Different guidelines exist for the treatment of HIV/AIDS which mainly include Centre for Disease Control (CDC), World Health Organization (WHO), British HIV association (BHIVA), HIV clinical guidelines programme, New York and National AIDS Control Organization (NACO), India. Though ART is recommended for all HIV-infected individuals in most of the guidelines, regardless of CD4 count, NACO India is yet to implement this policy. ART is also recommended for HIV-infected individuals to prevent HIV transmission (PreP).Patient education and counseling is very important before initiating ART to overcome the challenges with the improper use of ART and to maximize the benefits.
Challenges with the use of ARV Drugs
Newer anti-retroviral drugs
Following are the newer anti retroviral drugs which have shown promising result to combat the challenges for HIV infection (Figure 3).
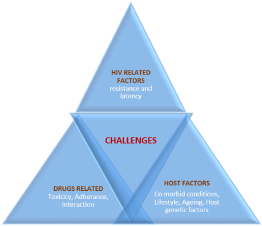
Figure 3: Challenges with the Use of ARV Drugs [6].
Tenofovir alafenamide
Other Names: GS-7340, TAF, TFV alafenamide, A prodrug of tenofovir, tenofovir alafenamide fumarate etc.
Drug Class: Nucleoside Reverse Transcriptase Inhibitors
Phase of Development [7]: The following TAF-containing FDC regimens are in Phase III studies:
1) emtricitabine/TAF 2) rilpivirine/emtricitabine/TAF 3) darunavir/cobicistat/emtricitabine/TAF , and 4) GS-9883/ emtricitabine/TAF and Emtricitabine/TAF and rilpivirine/ emtricitabine/TAF FDC tablets are currently under review by the U.S. Food and Drug Administration (FDA) for marketing approval.
Mechanism of action: TAF belongs to a class (group) of HIV drugs called nucleoside reverse transcriptase inhibitors (NRTIs). By blocking reverse transcriptase, NRTIs prevent HIV from multiplying and can reduce the count of HIV in the body.
Pharmacokinetic details
TAF is a prodrug. A prodrug does not work until the body converts it into an active form. In the body, TAF is converted to tenofovir diphosphate (TFV-DP). TAF is designed to circulate systemically as the prodrug and undergo conversion to tenofovir intracellularly, achieving higher active metabolite concentrations in peripheral blood mononuclear cells and lower plasma tenofovir exposures than Tenofovir Disoproxil Fumarate (TDF) does [8] (Table 2).
Parameters
Tenofovir Disoproxacil Fumarate (TDF)
Tenofovir Alafenamide
(TAF)
Clinical trial phase
Approved
Phase III
Dose
300 mg OD
25 mg OD with food
Protein binding
Very low: < 0.7% to human plasma proteins and < 7.2% to serum proteins
~80%
Half-life (T½)
The median terminal elimination half-life is approximately 17 hours.
The median terminal half-life is 0.51 hours. However the active metabolite, tenofovir diphosphate, has an intracellular half-life of 150 to 180 hours.
Metabolism
Cytochrome P450 enzyme system is not involved
Cytochrome P450 3A (CYP3A)-mediated metabolism of TAF is minor
Excretion
By IV administration, 70-80% of the dose is recovered in the urine as unchanged drug within 72 hours
Mainly excreted in Feces upto 31.7% and in urine ‹ 1%
Adverse Events
Rash, diarrhea, headache, pain, depression, asthenia, nausea, and nephrotoxicity.
diarrhea, upper respiratory tract infection, fatigue, nausea, and rash
Table 2: Comparison between tenofovir disoproxacil fumarate and tenofovir alafinamide.
The Drugs Approved after 2012 under the Class of Integrase Inhibitors (Table 3)
Parameters
Elvitegravir
Cabotegravir [9]
Dolutegravir [10,11]
Synonym/Other Names:
EVG
744 LA, CAB, GSK-1265744, GSK1265744, GSK744, GSK744 LA, GSK744 LAP, S-265744, S/GSK1265744, cabotegravir LA, cabotegravir sodium etc.
DTG, S/GSK 1349572 or 572
Drug Class:
Integrase Inhibitors
Integrase Inhibitors
Integrase Inhibitors
Molecular Weight:
447.88 g/mol
405.35 g/mol
441.36 g/mol
Pregnancy category:
B (No risk in nonhuman group)
Not available
B (No risk in non-human group)
Approval status:
EVG approved as fixed dose combination in 27 AUG 2012 as “Stribild” and as single pill formulation in 24 SEP 2014.
IIb
DTG is approved on 13 AUG 2013 for adult and children above 12 years of weight atleast 40kg or more.
Dosage form:
85mg and 150mg tablet formulation which always taken with food and should be used with combination with other HIV medicines.
CAB is available in Tablet formulation as Oral carbotegravir/oral CAB and in Parenteral formulation as long acting injection/ carbotegravir LA or CAB LA
Tablets: 50 mg oral.
Absorption:
Oral administration with food increases three fold absorption and reaches Cmax within 3-4 hours.
LA cabotegravir is readily absorbed following intramuscular and subcutaneous administration
Oral administration of DTG gives peak plasma concentrations 2 to 3 hours post-dose.
Protein binding:
EVG shows 98% protein binding.
High protein binding.
High protein bounding i.e. greater than or equal to 98.9%
Metabolism:
Mainly via Liver byCytochrome P450 (CYP)3Aenzyme
CAB is primarily metabolized via glucoronidation by UGT1A1 (main pathway) and UGT1A9 (minor pathway).
DTG Primarily metabolized via UGT1A1 with some contribution from CYP3A enzymes.
Half-life:
12.9 hr.
21 to 50 days for long-acting parentral [LAP] nanosuspension administered via intramuscular [IM] or subcutaneous [SC] injection
40 hours for oral dosing.
DTG has a terminal half-life of approximately 14 hours.
Excretion:
Mainly occur through liver 93% and renal 7%
CAB is eliminated in feces primarily as unchanged drug and in urine as a glucuronide metabolite.
Major route of excretion is feces followed by urine. (Feces up to 53% and in urine 18.9%)
Warning and Precaution:
Care should be taken If you are allergic to EVG or have liver problem and if pregnant or breast feeding condition.
Injection site reaction may be observed.
Hypersensitivity Reactions and fat Redistribution can be seen also Immune Reconstitution Inflammatory Syndrome(IRIS) is also observed. Contraindication: Co-administration with dofetilide is contraindicated that may become life threatening.
Side effects/ Adverse Events:
Immune reconstitution inflammatory syndrome (IRIS) and the common side effect is diarrhea
For oral route, most AE seen is Headache.
And for parenteral route is Injection Site Reaction(ISR) is predominately mild (93%) and Grade 1
Allergic reaction and abnormal liver function in patient infected with Hepatitis B or C
Storage:
Store at room temperature below 86°F (30°C).
-20°C for long term storage of dried powder and -80°C for short term storage of solution (as available from commercial supplier).
Store at room temp. 68 to 77°F (20 to 25°C)
Table 3: Comparison of drugs approved after 2012 under the class of integrase inhibitors.
The adjuvant with ART: Cobicistat
The anti viral drugs are basically used to target virus entities, However being non-specific sometimes and also having problems with metabolism due to host drug metabolizing genes makeup issues like non-adsorption and toxicity are of major concern. Cobicistat does not have any antiretroviral activity but it is a potent CYP inhibitor. Thus it increases the concentration of antiretroviral drugs by decreasing the metabolism. This in turn gives us an opportunity to use drugs which would be long acting at a lower dose. Cobicistat has been adapted in practice and has following characteristics.
Other Names: COBI, GS-9350 etc.
Drug Class: Pharmacokinetic Enhancers (CYP3A Inhibitors)
Approved Use: As a pharmacokinetic enhancer in combination with other ARV agents for the treatment of HIV-1 infection [12].
Molecular Weight: 776.03 g/mol
Molecular Formula: C40 H53 N7 O5 S2
Dosage form: Tablets: 150 mg orally with food However renal dose adjustment is necessary if creatinine clearance is less than 70ml/ min.
Mechanism of action: COBI is a potent inhibitor of Cytochrome P450 3A enzyme including important CYP3A4 subtype. It also inhibits intestinal transport proteins which also increases overall absorption of several ARV drugs.
Pharmacokinetic properties
Protein binding: 97 to 98 %.
Half-life: Terminal half-life is ~3 to 4 hr.
Indication and usage: COBI is a CYP3A inhibitor indicated to increase systemic exposure in combination with other ARV agents for the treatment of HIV-1 infection
Side effect: COBI with Atazanavir gives yellowing of eyes (Jaundice) and nausea. Also, severe allergic reaction and symptom of kidney problems are also observed.
Precaution: Safety and efficacy are not being established patient younger than 18 yr. also testing prior to Initiation of cobicistat is necessary because it decreases estimated creatinine clearance due to inhibition of tubular secretion of creatinine without affecting the actual renal glomerular function
Contraindication: Co-administration with tenofovir is not recommended due to renal toxicity.
Storage: Cobicistat is stored at 25°C
Combination HIV medicines contain two or more HIV medicines from one or more drug classes (Table 4).
Generic Name (Other names and acronyms)
Brand Name
FDA Approval Date
Abacavir, DTG , and Lamivudine
Triumeq
August 22, 2014
Atazanavir and Cobicistat
Evotaz
January 29, 2015
Darunavir and Cobicistat
Prezcobix
January 29, 2015
Elvitegravir, Cobicistat, Emtricitabine, and TAF
Genvoya
November 5, 2015
Emtricitabine, Rilpivirine, and TAF
Odefsey
March 1, 2016
Table 4: ARV drug combination and FDA approval dates.
Newer drugs for HIV in Phase 1 and Phase 2 trials: [adapted from 13] (Table 5).
Name
Class
Amdoxovir
Nucleoside Reverse transcriptase inhibitors
Lersivirine
Doravirine
NonNucleoside Reverse transcriptase inhibitors
Cenicriviroc
Entry Inhibitor
Ibalizumab
Monoclonal antibody, entry inhibitor
Beverimat
Maturation inhibitor
AMD070
CXCR4 inhibitors.
HGS004 is a human immunoglobulin (Ig)
G4 monoclonal antibody against CCR5
Apricitabine
NRTI that is active against the M184V mutation
PRO 140
CCR5 monoclonal antibody
Vicriviroc
CCR5 antagonist
Vivecon (MP-9055)
HIV maturation inhibitor
BMS-663068
binds directly to the gp120 , Attachment inhibitor
Active regardless of whether an HIV strain uses CCR5 or CXCR4 co-receptors.
Table 5: Newer drugs for HIV in phase 1 and phase 2 trials.
Conclusion
Worldwide target for HIV/AIDS is 90–90–90. This is an ambitious treatment target to help end the AIDS epidemic By 2020 [14], it includes 90% of all people living with HIV will know their HIV status, 90% of all people with diagnosed HIV infection will receive sustained ARV therapy, and 90% of all people receiving ARV therapy will have viral suppression. So, to achieve this goal we need to have more bullets in the form of new drugs which are less toxic and are more efficacious.
TAF had a reduced impact on renal function and bone mineralization as consistently demonstrated by multiple parameters during clinical studies. The low dose and improved safety profile of TAF also have implications for the effective treatment of HIV in resource-limited settings where regular safety monitoring is not possible. Cobicistat provides an alternative to Ritonavir as a pharmaco- enhancer for ARV therapy and as a component of Stribild [15]. It offers an effective, well-tolerated, integrase inhibitor-based single-tablet regimen for HIV treatment which is easy to since it would be long acting [16]. Low adherence leads to the development of resistance hence efforts are directed to test Inj. Carbotegravir depot preparation. HIV could be suppressed not eliminated hence adherence remains a critical issue. Prep trials which focus on HIV prevention among MSM, Heterosexual couples have shown some promising results with Inj. Carbotegravir [17].
New drug development efforts always try to bring in more efficacious and less toxic molecules. However, no drug is perfect. There are limitations like availability of platforms for bigger clinical trials with significant number of participants to look for adverse effects of the drug. Genetic variations in PK/PD owing to ethnicity are also not known in all the cases. The cost of these newer drugs when they would be approved by FDA are also unpredictable as lot of research effort and regulatory efforts have gone into it. Once available in the market, continuous uninterrupted supply of these drugs with affordable cost for patient or free of cost under National program also remain points of consideration. Another big issue is about the acceptability of the drug and adherence as per the prescribed dosages and times pan by the patients.
Though there are many challenges, efforts by many workers round the world say that we are hopeful with respect to the goal of 90–90–90 by 2020.
Acknowledgement
The authors would like to acknowledge National AIDS Research institute and Symbiosis international University, Pune for the encouragement and support during the preparation of this review article.
References
- Gallant JE. 100 Questions & Answers about HIV and AIDS. Jones & Bartlett Publishers. 2012.
- Antiretroviral drugs used in the treatment of HIV infection.
- Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012; 2: a007161.
- Kumarasamy N1, Patel A, Pujari S. Antiretroviral therapy in Indian setting: when & what to start with, when & what to switch to? Indian J Med Res. 2011; 134: 787-800.
- Guideline for initiation of anti retroviral drugs.
- Desai M, Iyer G, Dikshit RK. Antiretroviral drugs: critical issues and recent advances. Indian J Pharmacol. 2012; 44: 288-298.
- Sax PE, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, noninferiority trials. The Lancet. 2015; 385: 2606-2615.
- Ray AS, Fordyce MW, Hitchcock MJ. Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus. Antiviral Res. 2016; 125: 63-70.
- Andrews CD, Heneine W. Cabotegravir long-acting for HIV-1 prevention. Curr Opin HIV AIDS. 2015; 10: 258-263.
- Network HPT. HPTN 077 A Phase IIa safety, tolerability and acceptability study of an investigational injectable HIV integrase inhibitor, GSK1265744, for PrEP in HIV uninfected men and women.
- Greener BN, et al. Dolutegravir pharmacokinetics in the genital tract and colorectum of HIV-negative men after single and multiple dosing. J Acquir Immune Defic Syndr. 2013; 64: 39-44.
- Nathan B, Bayley J, Waters L, Post FA. Cobicistat: a Novel Pharmacoenhancer for Co-Formulation with HIV Protease and Integrase Inhibitors. Infect Dis Ther. 2013; 2: 111-122.
- Cited. 2016: mar 01.
- Hill A, Pozniak A. HIV treatment cascades: how can all countries reach the UNAIDS 90-90-90 target? AIDS. 2015; 29: 2523-2525.
- Mills A, Crofoot G, Ortiz R, Rashbaum B, Towner W, Ward D, et al. Switching from twice-daily raltegravir plus tenofovir disoproxil fumarate/emtricitabine to once-daily elvitegravir/ cobicistat/ emtricitabine/ tenofovir disoproxil fumarate in virologically suppressed, HIV-1-infected subjects: 48 weeks data. HIV Clin Trials, 2014; 15: 51-56.
- Shimura K, Kodama EN. Elvitegravir: a new HIV integrase inhibitor. Antivir Chem Chemother. 2009; 20: 79-85.
- DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006; 43: 1-5.