
Research Article
Austin J HIV/AIDS Res. 2016; 3(3): 1030.
Hepatitis C Virus (HCV) Genotype 3 is Associated with Higher Grade of Liver Fibrosis in Hepatitis C Virus Infected Patients
Kundu N¹, Gupta E¹*, Rastogi A², Kumar G³ and Khurana J¹
¹Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi, India
²Department of Clinical Pathology, Institute of Liver and Biliary Sciences, New Delhi, India
³Department of Research, Institute of Liver and Biliary Sciences, New Delhi, India
*Corresponding author: Gupta E, Department of Clinical Virology, Institute of Liver and Biliary Sciences, New Delhi, India
Received: September 20, 2016; Accepted: November 01, 2016; Published: November 03, 2016
Abstract
In Chronic hepatitis C (CHC), one of the most important causes of chronic liver diseases, exact association of viral factors such as Genotype and viral load with fibrosis is not yet determined.
Objective: To investigate relationship between HCV genotypes, viral load, biochemical markers and degree of liver fibrosis in patients with chronic HCV infection.
Materials and Methods: Retrospective analysis of 887 HCV positive patients was done. Liver biopsy was done in 154 patients and degree of fibrosis was evaluated by modified ISHAK scoring system. HCV viral load was determined by COBAS TaqMan HCV test v2.0 (Roche Molecular System Inc, Branchburg, NJ, USA) and genotyping was performed by Linear Array HCV Genotyping Test (Roche Molecular System Inc, Branchburg, NJ, USA).
Result: The mean age of study population was 47.63 (±15.08) years and male: female ratio was 2.68:1. Overall mean HCV viral load was 6.26x105 (±12.52) IU/ml, mean ALT level 62.65 (±2.16) IU/ml, mean AST level 43.0 (±2.03) IU/ml and mean total bilirubin level 1.00 (±0.19) mg/dl. Genotype 3 (69.4%) was most common genotype followed by 1 (25.6%) and 4 (4.6%). The biochemical markers (ALT, AST and total bilirubin) were significantly higher in patients with genotype 3 as compared to genotype1 (p=0.036, 0.000 and 0.012 respectively). Of 154 patients, fibrosis score <3 was seen in 96 (62.5%) patients and =3 in the remaining 58 (37.5%) patients. Genotype 3 was significantly correlated with higher (=3) fibrosis score (p=0.009).
Conclusion: Genotype 3 was found to be significantly associated with higher liver fibrosis which may have implications in clinical management of genotype 3 infected patients.
Keywords: Chronic hepatitis; Hepatitis C virus; Genotypes; Fibrosis
Abbreviations
HCV: Hepatitis C virus; CHC: Chronic hepatitis C; HBV: Hepatitis B Virus; HIV: Human Immunodeficiency virus; FPR: Fibrosis progression Rate; OPD: Outpatient; ICU: Intensive care units; HIS: Hospital Information System; CMIA: Chemiluminiscence Micropartice Immunoassay; RNA: Ribonucleic Acid; RT-PCR: Reverse transcription-polymerase chain reaction; CHB: Chronic Hepatitis B; ILBS: Institute of Liver and Biliary Sciences; FDA: Food and Drug Administration; ALT: Alanine Amino Transferase; AST: Aspartate Amino Transferase; SPSS: Statistical Package for Social Studies; IU/ml: international unit per millilitre; mg/dl: Milligram per Decilitre; HCC: Hepato-Cellular Carcinoma
Introduction
Approximately 3% of the world’s populations, (more than 350 million people) are chronically infected with hepatitis C virus (HCV) [1]. Chronic hepatitis C (CHC) is one of the most important causes of chronic liver diseases ranging from mild inflammation to fibrosis, cirrhosis and hepatocellular carcinoma, associated with the increased morbidity and mortality [2]. The identification of factors affecting fibrosis progression is critical for the optimal management of infected patients [3]. Factors associated with rapid progression of fibrosis include demographic characteristics (such as older age at infection and male sex), host genetic factors, viral co-infections (with the hepatitis B [HBV] or the human immunodeficiency virus [HIV]), metabolic features (such as steatosis, insulin resistance or iron overload) and exposure to toxic agents (alcohol, tobacco or cannabis) [4]. The estimation of fibrosis progression rate (FPR) based on the ratio of fibrosis stage to disease duration has been shown to reflect the true fibrosis progression. Recent studies have suggested that some viral genotypes, such as genotype 3, are associated with more rapid fibrosis progression than other genotypes [5-7].
It is well established fact that in HCV infected patients, the clinical findings, genotypes and viral load are strong predictors for the outcome of antiviral therapy [8]. Several authors tried to develop correlations between various non-invasive markers of liver damage (serum hyaluronic acid levels, collagen level, platelet count, serum bilirubin levels and elevated transaminases levels) with HCV viral load and genotypes in HCV infected patients, but no clear conclusions were formed [9-13].
This study was conducted to find the prevalence of HCV genotypes in Delhi, and to further investigate relation of these genotypes with liver fibrosis and disease activity markers. The correlation of genotype with severity of histopathological disease has not been studied from India, and only few studies have correlated viral load, biochemical markers with genotypes.
Materials and Methods
Patients
In the retrospective analysis, a total of 887 patients were enrolled according to inclusion/ exclusion criteria from the people visiting outpatient (OPD) or those admitted in wards/intensive care units (ICUs) of our hospital during January 2011 to July 2014. The liver biopsy was done only in 157/887 patients (according to the indication of biopsy as per institute’s protocol). The study was approved by the Ethics Committee of the institute. A detailed clinical history and clinical examination results were obtained from HIS (hospital information system) of the institute.
Inclusion criteria: Patient’s positive both for Anti HCV antibodies using 3rd generation Anti HCV chemiluminiscence micropartice immunoassay (CMIA) (Anti HCV Architect System, Abott, Weisbaden, Germany) and HCV Ribonucleic Acid (RNA) detection by reverse transcription-polymerase chain reaction (RTPCR).
Exclusion criteria: Patients with co-infections [chronic hepatitis B (CHB) or HIV], other non- infectious causes of chronic liver disease, history of alcohol intake, taking immunosuppressive drugs and chronic renal insufficiency were excluded from the study.
Samples
Peripheral blood (serum and plasma) samples were collected from each patient for enzyme immunoassays, the measurement of biochemical markers of liver damage and the investigation of viral ribonucleic acid (RNA) and HCV genotyping by molecular biology techniques. HCV serology and biochemical markers were tested using commercially available chemiluminiscent microparticle immunoassay method (CMIA) (Abott Laboratories, Chicago, IL, USA).
Quantitative measurement of hepatitis C viral load
Plasma sample collected from each patient was used to extract HCV RNA using high pure viral RNA extraction as per manufacturer’s instructions (Roche Diagnostic GmbH, Mannheim, Germany). The eluted RNA was stored at -70°C until use. HCV RNA load was determined by FDA approved COBAS TaqMan HCV test v2.0 (Roche Molecular System Inc, Branchburg, NJ, USA). The linear range of this real time PCR assay is 25IU/ml to 3.9x108 IU/ml and lower limit of detection is 25IU/ml.
HCV genotype analysis
Genotyping was performed by Linear Array HCV Genotyping Test (Roche Molecular System Inc, Branchburg, NJ, USA).
Histological evaluation of biopsy samples
The modified ISHAK scoring system was used to grade degree of fibrosis and histological activity in liver biopsy samples at department of Pathology, institute of Liver and Biliary Sciences (ILBS) [14]. Liver biopsies were evaluated by two independent pathologists without former information to patient’s history. Liver histological staging was based on six scores of fibrosis: as score 0 (no fibrosis), 1 (mild fibrosis of some portal areas without septa), 2 (mild fibrosis of most portal areas without septa), 3 (moderate fibrosis with occasional septa), 4 (fibrous expansion of portal areas with marked bridging [portal to portal (P-P) as well as portal to central (P-C)), 5(marked bridging (P-P and/or P-C) with occasional nodules (incomplete cirrhosis), 6(Cirrhosis, probable or definite). These scores were further grouped as 0(no fibrosis), 1-2 (mild fibrosis) and =3 (moderate fibrosis to cirrhosis) [14].
Association of HCV genotypes with viral and host factors
HCV genotypes were correlated with viral load, host biochemical markers (alanine aminotransferase (ALT); aspartate amino transferase (AST) and total bilirubin) and liver biopsy study results. Different markers were also correlated with fibrosis staging and histologic activity index.
Statistical analysis
The statistical analysis was performed using the statistical package for social studies (SPSS) version 17 for windows. Student t-test and Chi-square tests were applied to evaluate differences in proportions. P value <0.05 was considered significant. The normal values of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin level were (~5-40 IU/ mL), (~10-40 IU/ mL) and (< 1.0 mg/ dl) respectively. Multiple regression analysis was used to evaluate independent associations between HCV genotypes and individual demographic characteristics, biochemical values and viral load to identify variables association within different genotypes. For further analysis of different blood markers with respect to genotypes, only genotypes 1 and 3 were studied because of very small sample size in other two groups (using Mann-Whitney test). The correlation of serum markers and viral load was analyzed by Spearman’s correlation for non parametric data.
Results
Of the total 887 patients included in study, 646 (73%) were males while 241(27%) were females. The mean age of the study population was 47.63 (± 15.08) years. The most frequently detected genotype was 3 (69.4%) followed by 1 (25.6%) and 4 (4.6%). The genotype 2 was detected in only 3(0.3%) patients. The frequency distribution of different genotypes according to age and gender is given in Table 1.
Characteristics
Genotype 1
Genotype 2
Genotype 3
Genotype 4
Total (n=887)
227
(25.6%)
3
(0.3%)
616
(69.4%)
41
(4.6%)
Mean age (SD)
47.63
(±15.08)
45.91
(±12.5)
44.00
(±14.52)
47.93
(±13.43)
Age range (years)
19-73
24-68
12-69
23-59
Age groups (years)
<40 (n=250)
69
1
164
16
=40 (n=637)
158
2
452
25
Sex
Male (n646)
165
2
443
36
Female (n=241)
62
1
173
5
Table 1: Frequency distribution of different genotypes according to age and gender.
The overall mean HCV viral load was 6.26x105 IU/ml (±12.52) across all genotypes. The mean ALT level was 62.65 (±2.16) IU/ml, mean AST level 43.0 (±2.03) IU/ml and mean total bilirubin level was 1.00 (±0.19) mg/dl. The comparison of different biochemical markers and viral load with respect to various genotypes was done (Table 2). The HCV viral load was found to be higher in genotype 2 infected patients followed by genotypes 1, 3 and 4 respectively. The highest ALT levels were observed in genotype 3 infected patients. Total bilirubin levels were also higher in genotype 3. On multivariate analysis using Kruskal Wallis Test, we found AST as the only marker that was significantly distributed amongst different genotypes and was significantly higher in genotype 3 as compared to other genotypes.
Laboratory markers
Genotype 1
Genotype 2
Genotype 3
Genotype 4
p value
HCV RNA level
(IU/ml )
8.94 x105
(±12.53)
1.34 x106
(±14.55)
5.57 x105
(±12.85)
4.90 x105
(±6.68)
0.398
Total bilirubin
(mg/dl)
1.31(±1.82)
1.0(±1.0)
1.44(±2.31)
1.34(±1.75)
0.831
ALT levels
( IU/ml)
56.75(±2.38)
63.00(±1.42)
64.97(±2.10)
60.29(±1.69)
0.213
AST levels
(IU/ml)
61.96(±2.22)
55.74(±1.49)
79.25(±1.95)
59.09(±1.7)
0.003
Data were expressed as mean ± S.D. ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase.
Table 2: Comparison of different parameters with respect to genotypes.
On correlation of various parameters between two most prevalent genotypes 3 and 1, all blood markers were significantly different among two genotypes. The HCV viral load was significantly higher in patients with genotype 1 as compared to genotype 3 (p=0.015). All other biochemical markers (ALT, AST and total bilirubin) were significantly higher in patients with genotype 3 as compared to genotype1 (p=0.036, 0.000 and 0.012 respectively).
Correlation between biochemical markers and HCV viral load
Laboratory markers like ALT, AST and Bilirubin were correlated with HCV viral load. It was found that viral load has significant positive correlation with ALT (r=0.175, p=0.000) and a negative correlation with total bilirubin ((r=-0.261, p=0.000).
Association of HCV genotypes with liver fibrosis score
Liver biopsy was done in 154 patients as per clinicians request to assess the grade of fibrosis or cirrhosis. Fibrosis scoring was done as per modified ISHAK scoring system [14]. Distribution of genotypes is as follows: Genotypes 1, 2, 3 and 4 were identified in 41,2,96 and 10 patients respectively. Overall, 96 (62.5%) of patients had a fibrosis score (<3), and the rest (37.5%) had fibrosis score=3 (Table 3). Significant association of different genotypes was seen with fibrosis scoring (p=0.002). An analysis of the histopathological alterations according to viral genotype using Pearson Chi-Square test showed higher degree of fibrosis (score=3) among patients infected with genotype 3 as compared to genotypes 1, 2 and 4. Of all the blood markers, only AST showed significant correlation with fibrosis scoring (Figure 1. Box plot).
Fibrosis score
HCV genotypes
1
2
3
4
Total no
0
8(19.5%)
0(0.0%)
9(9.4%)
2(20%)
19(12.5%)
1,2
24(58.5%)
1(50%)
44(41.8%)
8(80%)
77(50%)
=3
9(22%)
1(50%)
48(50%)
0(0.0%)
58(37.5%)
Overall
41(100.0%)
2(100.0%)
96(100.0%)
10(100.0%)
154
Table 3: Comparison of liver fibrosis scores with respect to genotypes.
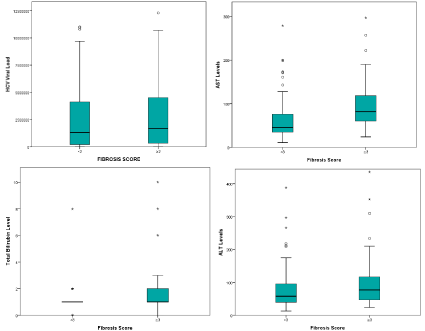
Figure 1: Box plot of AST, ALT, viral load and total bilirubin for fibrosis scoring. The horizontal line inside each box represents the median, while the top and bottom
of boxes represent the 25th and 75th percentiles, respectively. Vertical lines from the ends of the box encompass the extreme data points.
Discussion
The basic aim of our study was to find the prevalence of HCV genotypes in Delhi and to investigate relation of these genotypes with liver fibrosis and disease activity markers. The distribution of genotypes of Hepatitis C virus in our study has only reconfirmed that genotype 3 (69.4%) is the most common HCV genotype in Indian patients followed by genotype1 (25.6%). In study by Christdas J et al. 2013 from south India, HCV genotype 3 was found to be the most predominant (63.85%) followed by genotype 1 (25.72%), genotype 4 (7.5%), genotype 6 (2.7%) and genotype 2 (0.002%) [15]. Genotype 4 is being increasingly reported (41 patients in our study) and genotype 2 is rare in India (only 3 patients in our study) [15,16]. No genotype 6 or recombinants were found in our study. A definite male predominance and mean age being 47.63 (± 15.08) years show the trend of distribution of Indian hepatitis C patients; as has been documented in other studies [16-18]. No significant difference in distribution of various genotypes with respect to age or gender was found.
Raised serum transaminases are markers of Liver cell injury and cirrhosis is usually associated with elevated ALT levels. High bilirubin level has been shown to be associated with high likelihood of liver metastases and liver tumor involvement leading to hepato-cellular carcinoma (HCC) and liver cirrhosis by active or non- active HCV or HBV [19]. As different genotypes can lead to diverse severity of liver disease, the genotype detection might be useful in prevention of dreaded complication of CHC such as cirrhosis and HCC.
In our study, we found that genotype 3 has higher aminotransferases and bilirubin levels as compared to other genotypes as shown in table 2. This is in contrast to study by Ijaz et al. 2011 [13] and El-Zayadi A et al. 1996 [20], who showed that genotype4 is associated with higher aminotransferases level and higher risk of cirrhosis. Association of genotypes with different biomarkers and viral load has shown conflicting results in various studies and hence the correlation is controversial [11,13,14]. On comparison between genotype 3 and genotype 1 infected patients, we found significantly high bilirubin, ALT, AST and viral load levels in patients with genotype 3 as compared to genotype 1. This again favors that more liver damage is occurring in genotype 3 patients as compared to genotype 1, stressing again on higher fibrosis in genotype 3 infected patients. High viral replication might lead to hepatocyte damage and hence, higher ALT levels.
We further evaluated the association of genotypes with fibrosis staging. Patients infected with genotype 3 were found to have significantly higher degree of fibrosis as compared to patients with genotypes 1 and 4. Similar correlation of genotype 3 and liver fibrosis severity was shown by Hissar SS et al, 2006 [21] but not in other Indian study [22]. It was not possible for us to study the fibrosis progression rate as strict protocols are being followed for indicating liver biopsy. Our results are in keeping with other studies from world which observed a higher HAI score and higher portal, lobular or periportal inflammation or higher fibrosis progression rate in infection with 3a genotype as compared to other genotypes [23,24].
The treatment for HCV infection has taken another leap after introduction of newer drugs like Sofosbuvir, Ledipasvir or Daclatasvir alone or as a combination with Ribavirin or interferons. Though the efficacy of newer combination of drugs is found to be high (SVR>90%) for all the genotypes, the cost issues is a major hindrance to their worldwide use [25]. As genotype 3 is most common HCV genotype (>60% of HCV infected population) in India and it was found to be associated with higher fibrosis grade at time of diagnosis (in our study) as well with higher fibrosis progression rate, there is need of early treatment of all HCV infected patients to prevent cirrhosis or HCC. The protocols should be reviewed to start early treatment of all HCV infected patients and regular assessment should be done for prevention of HCC as higher grade of fibrosis is itself a risk factor for HCC. Patients should be made aware of the genotype, individualized counselling, with particular attention given to the controllable factors, such as alcohol consumption and overweight must be done.
The limitation of our study was that longitudinal studies could not be done to study the fibrosis progression rate in chronic HCV patients. Also, the study should be continued to confirm the results on large no. of patients.
References
- Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol. 2006; 44: S6–S9.
- Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001; 345: 41–52.
- Massard J, Ratziu V, Thabut D et al. Natural history and predictors of disease severity in chronic hepatitis C. J Hepatol. 2006; 44: S19–S24.
- Missiha SB, Ostrowski M, Heathcote EJ. Disease progression in chronic hepatitis C: modifiable and nonmodifiable factors. Gastroenterology. 2008; 134: 1699–1714.
- Hezode C, Roudot-Thoraval F, Nguyen S et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005; 42: 63–71.
- Hissar SS, Kumar M, Tyagi P et al. Natural history of hepatic fibrosis progression in chronic hepatitis C virus infection in India. J Gastroenterol Hepatol. 2009; 24: 581–587.
- Bochud PY, Cai T, Overbeck K et al. Genotype 3 is associated with accelerated fibrosis progression in chronic hepatitis C. J Hepatol. 2009; 51: 655–666.
- Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to Hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002; 36: 1266-1272.
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001; 357: 1069-1075.
- Al-Khurri LE, Al-Khafaji KR, Al-Salihi SA, Alwaysi SAA, Al-Akayshi RJ. Serum HCV-RNA levels in patients with chronic hepatitis C: Correlation with histological features. Arab J Gastroenterol. 2009; 10: 10-13.
- Zechini B, Pasquazzi C, Aceti A: Correlation of serum aminotransferases with HCV RNA levels and histological findings in patients with chronic hepatitis C: the role of serum aspartate transaminase in the evaluation of disease progression. Eur J Gastroenterol Hepatol. 2004; 16: 891-896.
- Ijaz et al. Association of laboratory parameters with viral factors in patients with hepatitis C. Virology Journal 2011; 8: 361.
- Ahmad et al. HCV genotype-specific correlation with serum markers: Higher predictability for genotype 4a. Virology Journal. 2011; 8: 293.
- Ishak KG. Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol. 2000; 113: 40-55.
- Christdas J, Sivakumar J, David J, Daniel H, Raghuraman S, Abraham P. Genotypes of hepatitis C virus in the Indian sub-continent: A decade-long experience from a tertiary care hospital in South India. Indian J Med Microbiol. 2013; 31: 349-353.
- Raghuraman S, Abraham P, Sridharan G, Ramakrishna BS. Hepatitis C virus genotype 6 infection in India. Indian J Gastroenterol. 2005; 24: 72-73.
- Abraham R, Ramakrishna B, Balekuduru A, Daniel HDJ, Abraham P, Eapen E, Kurian G. Clinicopathological features and genotype distribution in patients with hepatitis C virus chronic liver disease. Indian J Gastroenterol. 2009; 28: 53-58.
- Chakravarti A, Dogra G, Verma V, Srivastava A P. Distribution pattern of HCV genotypes & its association with viral load. Indian J Med Res. 2011; 133: 326-331.
- Raymond E, Boige V, Faivre S, Sanderink GJ, Rixe O, Vernillet L, et al. Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol. 2002; 20: 4303-4312.
- El-Zayadi A, Simmonds P, Dabbous H, Prescott L, Selim O, Ahdy A. Response to interferon-alpha of Egyptian patients infected with hepatitis C virus genotype 4. J Viral Hepat. 1996; 3: 261-264.
- Hissar SS, Goyal A, Kumar M, Pandey C, Suneetha PV, Sood A, et al. Hepatitis C virus genotype 3 predominates in North and Central India and is associated with significant histopathologic liver disease. J Med Virol. 2006; 78: 452-458.
- Singh S, Gupta R, Malhotra V, Sarin SK. Predictors of histological activity and fibrosis in chronic Hepatitis C infection: A study from North India. Indian J Pathol Microbiol. 2010; 53: 238-243.
- Reiberger T, Ferlitsch A, Sieghart W, Kreil A, Breitenecker F, et al. HIV-HCV co-infected patients with low CD4+ cell nadirs are at risk for faster fibrosis progression and portal hypertension. J Viral Hepat. 2010; 17: 400-409.
- Probst A, Dang T, Bochud M, Egger M, Negro P and Bochud PY. Role of Hepatitis C virus genotype 3 in liver fibrosis progression - a systematic review and meta-analysis J. Viral Hepatitis. 2011; 18: 745-759.
- Webster D P, Klenerman P & Dusheiko GM. Lancet Seminar – Hepatitis C. Lancet (London, England). 2015; 385: 1124-1135.