
Research Article
Austin J HIV/AIDS Res. 2022; 8(1): 1053.
Prevalence of Pubertal Delay in Paediatric Human Immunodeficiency Virus Infection
Kan KM1*, Muyele H1, Monono N2, Fomenky C1, Chiabi A1 and Mah E3
¹Department of Clinical Sciences, Faculty of Health Sciences, The University of Bamemda, Cameroon
²Department of Paediatrics, Faculty of Health Sciences, University of Buea, Cameroon
³Department of Paediatrics, Faculty of Medicine and Biomedical Sciences, University of Yaounde I, Cameroon
*Corresponding author: Kan KM, Department of Clinical Sciences, Faculty of Health Sciences, The University of Bamenda, North West Region, Cameroon
Received: October 04, 2022; Accepted: November 04, 2022; Published: November 11, 2022
Abstract
Background: In Cameroon, Paediatric HIV occurs in 7 per 500,000 people living with HIV. HIV infection can result in impaired sexual development and delayed puberty. There are few studies on the impact of HIV on puberty.
Aim: To determine prevalence of pubertal delay in paediatric population infected with Human Immunodeficiency Virus (HIV) on antiretroviral therapy.
Methods: This was a cross-sectional descriptive study that assessed puberty using the Marshall and Tanner staging system in 120 HIV positive children aged 8-18years, at the Bamenda Regional Hospital treatment Centre from February to May 2022. Data obtained included sex, age, tanner stage of development and age at puberty.
Results: Of 120 participants included, the majority 64(53.3%) were girls. The median (IQR) age was 13.9(11.1-15.8) and 71(59.2%) were aged =13years. Sixty-three (52.5%) participants were in Tanner stage 1-2 and the prevalence of pubertal delay was 12.7%. Median age at puberty in years was thelarche: 12.5(9.9-14.4), adrenarche: 13.2(11.8-14.6), menarche: 14(13-14) in girls and gonadarche: 12.9(11.6-14.4), adrenarche: 13.3(11.4-14.4) in boys.
Conclusion: About 13% of HIV infected children on ART experience pubertal delay with a prevalence rate of 12.7%.
Keywords: Puberty; Human immunodeficiency virus; Paediatrics
Introduction
Human Immunodeficiency Virus (HIV) in the paediatric population contributes to the high burden of infection in children aged 1-14 years and accounts for 1.7 million cases and 150,000 new infections annually with the Sub-Saharan Africa most affected [1]. In Cameroon, paediatric HIV occurs in 7 percent of 500,000 people who are living with HIV [2]. Morbidity and mortality from thisinfection is high and results in multi-systemic involvement including the endocrine system. Onset of puberty heralds sexual development and is enhanced by sex hormones bringing about body changes and selfimage. HIV infection results in impaired physical growth, wasting and resultant hypofunction of hypothalamic-pituitary gonadal axis resulting in impaired sexual development and delayed puberty [3-11]. Delayed puberty refers to lack of features of pubertal development by the age of 13 years in females and 14 years in males [13,14]. There are few studies on impact HIV on puberty and in Cameroon there is limited data on puberty in the paediatric HIV population, hence the necessity of this study to determine the prevalence of pubertal delay in a paediatric HIV infected population on Antiretroviral Therapy (ART) at a treatment centre in Cameroon.
Methods
Study Design and Setting
This was a hospital-based cross-sectional descriptive study over a 4 months period, carried out in the paediatric treatment centre of the Bamenda Regional Hospital (BRH); a second-level reference health institution for the North West Region of Cameroon. This facility follows up over 500 children aged between 0-19 years living with HIV and receiving Antiretroviral Therapy (ART) of which 250 are aged 8-18 years.
Study Population
Children aged 8-18 years who were HIV infected and followed up at the paediatric treatment centre of BRH whose parent or guardian consented to the study and who gave assent to participate in the study were included while those with chronic illnesses or genetic abnormalities such as sickle cell disease, congenital heart disease, chronic renal failure, diabetes, inflammatory bowel diseases, paediatric malignancies, genetic diseases (Downs syndrome, Turners syndrome) were excluded.
Ethical Consideration
Ethical clearance was obtained from the Institutional Review Board of the Faculty of Health Sciences, University of Bamenda and administrative authorization was also gotten from the North West Regional delegation for public health and the Bamenda Regional Hospital. Informed consent from the caregivers and assent from the children.
Study Procedure
Data Collection
Socio-demographic data was obtained in a structured preformed questionnaire and records of the physical examination to demonstrate features of sexual maturity. Sexual maturation was assessed by physical examination with the aid of graphic representations of the Marshall and Tanner staging system. This staging system grades pubertal development via assessment of external primary and secondary sexual characteristics in the subjects on a 5point ordinal scale in both boys and girls that ranges from 1 (pre pubertal) to 5 (adult like) for female breast, male genitalia and pubic hair for both males and females. Female participants were asked about their age at attainment of menarche.
Data Analysis and Management
Data obtained was entered into the software statistical package for social sciences (SPSS) version 26.0 for analysis. Quantitative variables were expressed as mean ± SD for normal distribution and medians and Interquartile Range (IQR) for non-normal or skewed distribution. Categorical variables were expressed as frequency and percentages.
Results
General Characteristics of Study Participants
Table I highlights the socio-demographics of the 120 enrolled participants consisting of 64 (53.3%) female and 56 (46.7%) male with a female to male ratio of 1.1.Most of the subjects were above 13 years (59.2%) followed by those aged [10-13] years constituting 28.3% while those aged 8 to less than 10 years were the minority.
Variable
Frequency(n)
Percentage(%)
Age groups
8 to <10years
15
12.5
10 to <13years
34
28.3
=13years
71
59.2
Median(IQR) age
13.9(11.1-15.8)
Gender
Male
56
46.7
Female
64
53.3
Age distribution in males(N=56)
8 to <10years
5
8.9
10 to <14years
26
46.4
=14years
25
44.6
Age distribution in females(N=64)
8 to <10years
10
15.6
10 to <13years
16
29.7
=13years
38
54.7
IQR interquartile range
Table 1: Sociodemographic profile of participants and guardians (N=120).
Tanner Staging in Males and Females
Table II represents puberty in females and males by age groups using breast and genitalia development respectively. Three out of 27 (11.1%) girls were in Tanner stage 1-2 for breast development in those aged 13 years or older while five out of thirty-six (19.9%) boys aged 14 years or older were in Tanner stage 1-2 of genitalia development. Amongst those with advanced puberty, very few females 2(5.4%) were aged between 10 and 13years and there were no males aged 10- 14 years in advanced stages of puberty.
Tanner stage
Age groups n(%)
Delayed
n(%)8 to<10years
10 to <13years*/
10 to <14years**=13years*/
=14years**Breast development (N=64)
B 1-2
10(37.0)
14(51.9)
3(11.1)
3(4.7)
B 3-5
0(0)
2(5.4)
35(94.6)
Genitalia development(N=56)
G 1-2
5(13.9)
26(72.2)
5(13.9)
5(8.9)
G 3-5
0(0)
0(0)
20(100)
* for breast development, **for genitalia development
Table 2: Tanner stages by age group in boys and girls (N=120).
In table III, the median (IQR) age in years at Tanner stage 2 for breast and pubic hair development was 12.5 (IQR 9.9-14.4) and 13.2 (IQR 11.8-14.6) in girls. More than half of the girls 33(50.8%) had not attained menarche. The median (IQR) age at menarche was 14.0(13.0-14.0) years. The median (IQR) age in years at Tanner stage 2 of genitalia and pubic hair development were 12.9(11.6-14.4) and 13.3(11.4-14.7) years respectively in boys.
Tanner stage
Frequency(percentage)
Age median(IQR)/years
GIRLS (N=64)
Breast development(B)
B1
22(34.4%)
10.5(9.1-11.4)
B2
5(7.8%)
12.5(9.9-14.4)
B3
11(17.2%)
14.3(13.2-15.2)
B4
12(18.7%)
15.3(14.8-15.8)
B5
14(21.9%)
17.2(16.2-17.7)
Pubic hair development(PH)
PH 1
23(35.9%)
10.5(9.0-11.3)
PH 2
4(6,3%)
13.2(11.8-14.6)
PH 3
10(15.6%)
14.2(12.9-15.7)
PH 4
14(21.9%)
15.2(14.9-15.9)
PH 5
13(20.3%)
17.2(16.5-17.7)
Menarche
31(49.2%)
14.0(13.0-14.0)
BOYS (N=56)
Genitalia development(G)
G1
24(42.9)
11.2(10.1-13.2)
G2
12(21.4)
12.9(11.6-14.4)
G3
3(5.4)
15.9(14.9-15.8)
G4
10(17.9)
15.6(15.5-16.7)
G5
7(12.5)
17.3(16.3-17.5)
Pubic hair development(PH)
PH 1
25(44.6)
11.2(10.1-13.2)
PH 2
11(19.6)
13.3(11.4-14.7)
PH 3
2(3.7)
15.7(14.9-15.7)
PH 4
11(19.6)
15.6(15.5-16.7)
PH 5
7(12.5)
17.2(16.3-17.5)
IQR interquartile range
Table 3: Median Age at Tanner stages and menarche in girls (N=120).
Pubertal Delay
Pubertal delay was defined as the presence of Tanner stage 1 or Tanner stage 2 at an age ≥ 2SD from the expected mean for Tanner stage 2 breast and genitalia development, which is 13years in females and 14years in males [7,8]. Using these cut-off ages, sixty-three participants, who accounted for 52.5% of the study population were assessed for delayed puberty. The overall prevalence of pubertal delay was 12.7% (95% CI: 4.5-20.9) as shown in figure 1. The prevalence in girls was 7.9% (3/38) and 20% (5/25) in males.
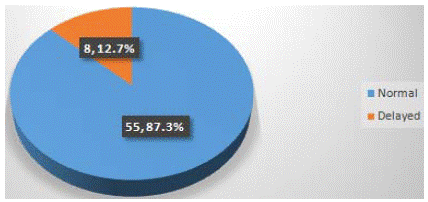
Figure 1: Prevalence of pubertal delay in study population.
Discussion
The prevalence of pubertal delay in our study population was 12.7%; with majority of boys (20%) experiencing delayed puberty compared to 7.5% in girls. Our results are higher than the 8.7% and 4.1% reported by Nana and collaborators in Uganda and Williams et al in the USA respectively [9,10]. Nana and colleagues only included patients on PI-boosted second line antiretroviral therapy [10] while Williams and colleagues studied a mixed racial population in a country with high living standards and consequently better nutrition and growth; different from our study involving subjects on other non PI based regimens and a cluster of purely black race in a developing country in a region plagued with war, poverty and food scarcity. Contrarily, McHugh et al found an even higher prevalence of 21% in HIV infected adolescents in Zimbabwe [11]. They had gender specific prevalence of 27 per 100 in girls and 13 per 100 in boys [11,12]. This higher prevalence could be because their participants had no prior antiretroviral therapy, whereas, in our study, all participants were already on ART for an average duration of 8years buttressing the benefits of antiretroviral therapy in infected children. Pubertal delay was more common in boys in our study which could be because of the usual earlier onset of puberty in girls regardless of their HIV serological status. The prevalence of pubertal delay in our study population amongst girls was comparable to the prevalence of 6.25% obtained in Nigeria by Iloh et al, [3] which could be explained by similarity in methodology. However Mbono et al in Yaounde, in Cameroon; among a similar population did not observe delay pubertal delay [13] despite similarities in study method.
The age of attainment of puberty in Cameroon among normal non-HIV infected children was reported at 8.9 years for girls and 9.6 years for boys [14]; which is earlier than the 12.5 years and 12.9 years respectively noted in our study population. (highlighting the impact of the disease on puberty). The age at onset of puberty in girls and boys in our study was similar to those of other authors in Africa and Europe [3,5,6] but different from Mbono et al and Buchacz et who found an earlier age at puberty [4,13]. Our observations may be due to the fact that majority of our subjects were in advanced stage of the disease at the time of diagnosis which may have been associated with an increase in the viral load and further depletion of immune function, increasing susceptibility to infections and increasing inflammatory markers; hence negatively affecting nutrition via decreased intake and poor absorption leading to impaired growth and resultant impairment of pubertal development via leptin hyposecretion [16-18] as seen with most chronic diseases [16,19,20].
Conclusion
The age at onset of puberty in the paediatric HIV population on ART in our study was 12.5 years in females and 12.9years in males with the prevalence of pubertal delay of 12.7% which was high. More males than females experienced delayed puberty.
Acknowledgements
We acknowledge all the children living with HIV on treatment who agreed to participate in our study and the staff of the Bamenda Regional Hospital treatment center caring for these children.
References
- Joint united nations programme on HIV/AIDS. UNAIDS data 2021. UNAIDS; 2021. Available from: https://www.unaids.org/sites/default/files/media_asset/ JC3032_AIDS_Data_book_2021.
- Joint united nations programme on HIV/AIDS. Country factsheet Cameroon 2020. UNAIDS; 2020. Available from: https://www.unaids.org/en/ regionscountries/countries/cameroon
- Iloh ON, Iloh KK, Ubesie AC, Emodi IJ, Ikefuna AN, Ibeziako NS. Comparison of Tanner staging of HIV-infected and uninfected girls at the University of Nigeria Teaching Hospital, Ituku/Ozalla, Enugu, Nigeria. Journal of Pediatric Endocrinology and Metabolism. De Gruyter; 2017; 30: 725–9.
- Buchacz K, Rogol A, Lindsey J, Wilson C, Hughes M, Seage G, et al. Delayed Onset of Pubertal Development in Children and Adolescents with Perinatally Acquired HIV Infection. Journal of acquired immune deficiency syndromes (1999). 2003; 33: 56–65.
- Mbwile G, Kisenge R, Naburi HE, Muze K. Pubertal Development among HIV-Infected Children aged 8-18 Years in Dar es Salaam, Tanzania: A Cross Sectional Study. Tanzania Medical Journal. 2021; 32: 1–17.
- Martino M de, Tovo P-A, Galli L, Gabiano C, Chiarelli F, Zappa M, et al. Puberty in perinatal HIV-1 infection: a multicentre longitudinal study of 212 children. AIDS. 2001; 15: 1527–34.
- Marcdante KJ, Kliegman RM. Nelson essentials of pediatrics. Eighth edition. China: Elsevier. Philadelphia, PA; 2019.
- Davidson S. Davidson’s principles and practice of medicine. 22nd ed. Walker BR, Colledge NR, Ralston S, Penman ID, Britton R, editors. Edinburgh ; New York: Churchill Livingstone Elsevier; 2014.
- Willams PL, Abzug MJ, Jacobson DL, Wang J, Van Dyke RB, Hazra R, et al. Pubertal Onset in HIV-infected Children in the Era of Combination Antiretroviral Treatment. AIDS. 2013; 27: 1959–70.
- Nana Jacqueline N, Musoka P, Bakeera-Kitaka S, Paul Kisitu G. Prevalence and factors associated with delayed puberty among adolescents on boosted protease inhibitor based second line art at PIDC: Makere University; 2018.
- McHugh G, Rylance J, Mujuru H, Nathoo K, Chonzi P, Dauya E, et al. Chronic Morbidity Among Older Children and Adolescents at Diagnosis of HIV Infection. Journal of Acquired Immune Deficiency Syndromes (1999). Wolters Kluwer Health; 2016; 73: 275.
- Williams PL, Jesson J. Growth and Pubertal Development in HIV-Infected Adolescents. Curr Opin HIV AIDS. 2018; 13: 179–86.
- Mbono RC, Sap Ngo Um S, Edongue M, Ndombo PK. Does HIV infection affect growth and puberty of Cameroonian children? Archives de Pédiatrie. 2021; 28: 238–41.
- Sap S, Komba D, Sobngwi E, Obama MT, Koki PO, Mbanya JC. Age of Onset of Puberty in Yaounde, Which Normative Reference Data? European Society of Paediatric Endocrinology Abstracts. Paris, France: Bioscientifica; 2016. 557.
- Szubert AJ, Musiime V, Bwakura-Dangarembizi M, Nahirya-Ntege P, Kekitiinwa A, Gibb DM, et al. Pubertal development in HIV-infected African children on first-line antiretroviral therapy. AIDS (London, England). Wolters Kluwer Health. 2015; 29: 609.
- Sperling M, editor. Pediatric endocrinology. 4th ed. Philadelphia, PA: Saunders Elsevier Inc; 2014.
- Kiess W, Reich A, Meyer K, Glasow A, Deutscher J, Klammt J, et al. A Role for Leptin in Sexual Maturation and Puberty?HRP. Karger Publishers. 1999; 51: 55–63.
- Clayton PE, Gill MS, Hall CM, Tillmann V, Whatmore AJ, Price DA. Serum leptin through childhood and adolescence. Clinical Endocrinology. 1997; 46: 727–33.
- Pozo J, Argente J. Delayed puberty in chronic illness. Best Practice & Research Clinical Endocrinology & Metabolism. 2002; 16: 73–90.
- Kao KT, Denker M, Zacharin M, Wong SC. Pubertal abnormalities in adolescents with chronic disease. Best Practice & Research Clinical Endocrinology & Metabolism. 2019; 33: 101275.