Review Article
Austin J Hydrol. 2014;1(1): 9.
Study Progress in Riverine Phytoplankton and its Use as Bio-Indicator - a Review
Naicheng Wu*, Britta Schmalz and Nicola Fohrer
Department of Hydrology and Water Resources Management, Kiel University, Germany
*Corresponding author: Naicheng Wu, Department of Hydrology and Water Resources Management, Institute for Natural Resource Conservation, Kiel University, Kiel 24118, Germany
Received: June 01, 2014; Accepted: July 21, 2014; Published: July 26, 2014
Abstract
The value of algae as bio-indicators has already been recognized in the mid of 19th century, however, little attention has been paid to the application of phytoplankton in ecological evaluation of rivers. In this review, we found that studies of phytoplankton showed a long-term increasing trend from 1961 to 2014. However, most of these studies were carried out in oceans, coastal areas, gulfs, lakes and reservoirs, while very few of them (14%) focused on riverine phytoplankton. As well as modeling studies, the utilizations of riverine phytoplankton as bio-indicator are still poorly investigated and the available few studies were those mainly published after the year of 2000. Therefore, we describe 28 algal indices of riverine phytoplankton potentially used for bio-assessment, which belong to community index, growth form, diversity index and biotic index. We also elucidate the calculation and classification methods of 5 common indices proposed in 1950s (Shannon's diversity index, saprobity index) and nowadays (trophic diatom index, Q index and phytoplankton index). Finally, four future directions and applications of riverine phytoplankton research were discussed and proposed: 1) standardization of sampling methods, 2) relations with environmental factors, 3) bio-indication and 4) modeling and predicting dynamics of riverine phytoplankton.
Keywords: Algal metrics; Bio-assessment; Environmental variables; Modeling; Riverine phytoplankton
Introduction
Phytoplankton (mainly planktonic algae), together with benthic algae and macrophytes, constitute the autochthonous primary producers in aquatic ecosystems and form part of the basis of the food web in terms of energy and material input [1]. Due to their short life cycle, planktonic algae respond quickly to environmental changes and are thus a valuable indicator of water quality [2-5] with the aim of effective water resources management and water pollution control. The value of algae as bio-monitor and bio-indicator for human disturbances (e.g. point and diffuse pressures, etc.) has already been recognized in the mid 19th century: the first concept which has been developed was the system of saprobity, which was mainly designed for organic pollution of streams and rivers [6,7]. Moreover, unlike fish and macroinvertebrates, algal communities are usually present before disturbance and generally persist in some form after disturbances. Therefore, application of algal indicators to assess rivers is increasing [8-11]. Recently, diatoms were used as a tracer of water source and hydrological connectivity in the mountainous Attert catchment [12]. The preliminary result of [13] showed that diatoms can help to detect the onset/cessation of surface runoff. Suggested for a meso-scale catchment [14,15] suggested that diatoms could reflect the geographic origin of stream water at the catchment outlet. However, compared to the numerous investigations in lentic water bodies (e.g. oceans, gulfs, lakes and reservoirs) little attention has been paid to the application of the phytoplankton in ecological evaluation of rivers [8].
In this study, by reviewing international scientific literatures, we described the long-term trends of phytoplankton research from past to 2014, with emphasis on riverine phytoplankton. We then summarized the algal indices widely used now for riverine bio-assessment. Based on our reviewed literatures, we finally proposed four possible future directions and applications of riverine phytoplankton research.
Methods and Summary of Literature Reviewed
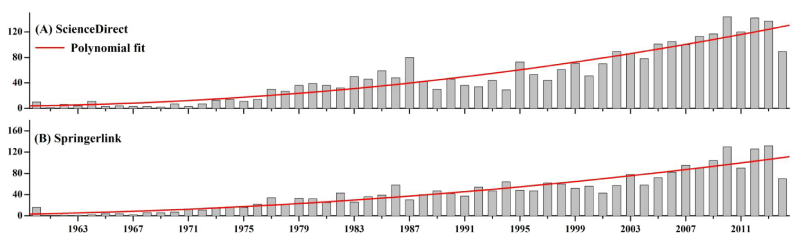
We searched original papers about phytoplankton by means of Science Direct: https://www.sciencedirect.com/ and Springer link: https://www.springerlink.com/ to inspect the long-term publication trends from 1961 (very few publications before 1960) to 2014 (access on 15th May, 2014). Publications with an article title of "phytoplankton" or "potamoplankton" were searched. The results showed that most of these studies were widely carried out in oceans, coastal areas, gulfs, lakes and reservoirs, and demonstrated an increasing publication trend by the two databases (Figure 1).
Figure 1: Long-term publication trends of phytoplankton studies searched by (A) ScienceDirect and (B) Springerlink (with article title of "phytoplankton" or "potamoplankton"). Red bold lines are study trends made by polynomial fit.
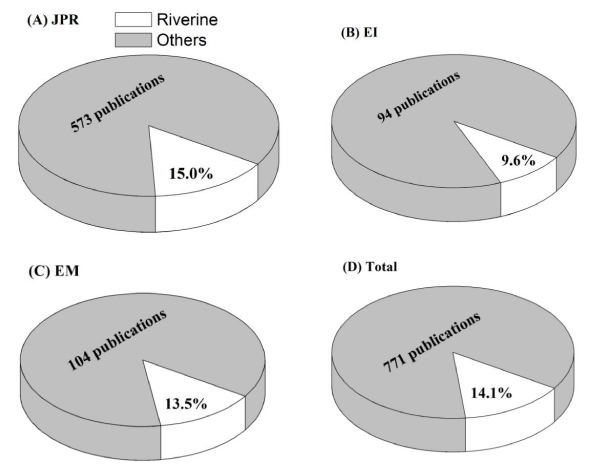
Based on the previous searching results, we conducted an additional search to estimate the proportion of riverine phytoplankton studies. We took three journals for in depth analysis, which were "J. Plankt. Res. (JPR)", "Ecol. Indic. (EI)" and "Ecol. Model. (EM)", respectively. For the period reviewed, we examined a total of 771 publications (with an article title of "phytoplankton" or "potamoplankton"), and only 14.1% (109 studies) of them focused on riverine phytoplankton (Figure 2). The proportions of riverine phytoplankton studies compared to other surveys of phytoplankton from JPR, EI and EM were 15.0%, 9.6% and 13.5%, respectively (Figure 2).
Figure 2: The proportions of riverine phytoplankton studies compared to other surveys of phytoplankton from (A) JPR (J. Plankt. Res.), (B) EI (Ecol. Indic.), (C) EM (Ecol. Model.) and (D) Total (sum of the three journals).
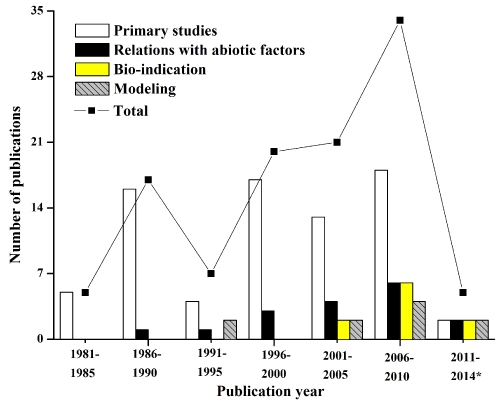
Furthermore, we classified these 109 studies into four major categories: I) primary studies (including taxonomic composition, temporal and spatial distribution, bio-volume, sampling methods, etc.), II) relations with abiotic factors, III) bio-indication and IV) modeling. We examined studies in five year increments (Figure 3). Overall, the number of riverine phytoplankton publications increased from 5 (first 5-y, 1981-1985) to 34 (the last 5-y, 2006-2010), except for a small number of 1991-1995 and 2011-2014. Most studies of them so far, however, were primary studies with a percentage of 68.8% (75 out of 109). There were only 17 publications (15.6%) studying "relations with abiotic factors", but an increasing trend was found from 1986-1990 (1 publication) to 2006-2010 (6 publications). As well as modeling studies, the utilization of riverine phytoplankton as bio-indicator was still poorly investigated and available studies were those mainly published after the year of 2000 (Figure 3).
Figure 3: Long-term publication trends of riverine phytoplankton studies in J. Plankt. Res. (JPR), Ecol. Indic. (EI) and Ecol. Model. (EM) was grouped in 5-y increments. Studies were classified into primary studies, relations with abiotic factors, bio-indication and modeling (see text). The total number of publications was also indicated for each period. * access on 15th May 2014.
Review of Algal Indices
Monitoring of the naturally occurring algal communities in rivers provide data on species composition, number, diversity or quantitative occurrence of the phytoplankton. However, experts of administrative institutions who are responsible for water quality management need simple numerical values rather than species lists or scientific evaluation of the assemblages [8]. In this section, therefore, we summarized 28 algal indices of riverine phytoplankton with potential to be used for bio-assessment (Table 1), which belong to community index, growth form, diversity index and biotic index. We emphasized the importance of algal bio-volume, and discussed calculation and classification methods of five common indices proposed in 1950s (Shannon's diversity index (H'), saprobity index (SaI)) and recent years (trophic diatom index (TDI), Q index (QI) and phytoplankton index (PhI)).
Index (R)
Abbreviation
Description
Reference
Community indices
Total algal biomass (V)
TAB
Measures total algal biomass per liter, and is estimated based on multiplication of density data with volume (closest geometric form) supposing specific gravity of 1.00 g cm-3
[16]
Total algal density (V)
TAD
Measures algal numbers per liter
-
Chlorophyll a Content (V)
Chl a
Measure total algal biomass
[103]
Ash-free dry-weight (V)
AFDW
[104]
Autotrophic index (+)
AI
Measures trophic status (autotrophic vs. heterotrophic) in rivers
[105]
Growth form indices
%benthic taxa (V)
BeT
Algal Data Analysis System (ADAS) using an attribute file of published values [27,23,28]
%mobile taxa (V)
MoT
%unattached taxa (V)
UnT
Diversity indices
Shannon's diversity index (-)
H'
Measures ecological diversity in the community
[21]
Menhinick index (-)
MeI
[105]
J1
[107]
Sheldon's evenness index2 (-)
J2
[108]
Evenness index3 (-)
J3
[109]
Evenness index4 (-)
J4
Evenness index5 (-)
J5
Camargo's evenness index6 (-)
J6
[110]
Simpson's dominance index (V)
D
[109]
Margalef's diversity index (V)
M
[111]
Species richness (V)
S
Number of specific or sub-specific taxa
-
Biotic indices
Trophic diatom index (+)
TDI
Designed to detect eutrophication
[33]
Saprobity index (+)
SaI
Measures saprobic status of the water
[26]
Q index (-)
QI
A new evaluation technique of potamoplankon for the assessment of the ecological status of rivers
[8]
Phytoplankton index (+)
PhI
New German approach to assess running waters by phytoplankton community
[39]
Assessment value of total pigment (+)
A-valuetotalpigment
Pennales-Index (-)
PeI
Chlorophyte-Index (+)
ChI
Cyanobacteria-Index (+)
CyI
Trophic index of potamoplankton taxa (+)
TIP
Table 1: Algal indices, their descriptions, and their expected response (R) to deterioration of water quality. + = indices expected to increase with deterioration, - =indices expected to decrease with deterioration, V = variable response.
Algal bio-volume and biomass
Algal bio-volume was commonly calculated to assess the relative abundance (as biomass or carbon) of co-occurring algae varying in shape and / or size [16]. We highlighted algal bio-volume because it was basis for calculations of many other indices, such as growth form index, diversity index, biotic index (Table 1). To describe the whole community, as many algal indices as possible should be calculated based on not only cell density, relative cell density, entity density (numbers of colonies, filaments or free-living cells), but also bio-volume or relative bio-volume [11].
In general, the calculation of bio-volume is based on geometric approximations. The geometric shapes used for bio-volume determination should be as close to the real shape of the organism but at the same time easily discernible and conveniently measurable during routine analysis [17]. [16] recommended a standard set of 20 geometric shapes for over 850 genera and provided equations to be used for accurate estimates of cell volume for phytoplankton and micro benthic algae from linear dimensions measured microscopically. Based on this earlier work, [18] proposed a set of 31 geometric shapes and equations of 284 phytoplankton genera for routine analysis in China Sea waters. Furthermore, a set of geometric models was suggested by [19] for calculating the cell bio-volumes of 201 phytoplankton genera found in transitional water ecosystems of the Mediterranean Ecoregion. The equations were designed to minimize the effort of microscopic measurements. [11] provided geometric shapes for 303 taxa in three mid-continent US great rivers (the Upper Mississippi, Missouri and Ohio). After calculating the algal bio-volume, biomass of each algal taxon was estimated based on multiplication of cell density data with volume (closest geometric form) supposing specific gravity of 1.00 g cm-3 [16,20]. Total algal biomass (TAB) is calculated by summing up the biomass of each tax on.
Shannon's diversity index (H') [21]
Shannon's diversity index (H'), based on information theory, is one of several diversity indices used to measure diversity in categorical data. The advantage of this index is that it takes into account the number of species and the distribution of the species. The index is increased either by having additional unique species, or by having a greater species distribution. Its equation is:
where:
H' = the Shannon's diversity index
Pi = proportion of all individuals in sample that belong to species i
S = total number of species in a sample
Σ = sum from species 1 to species S
H' was the most popular diversity index among ecologists [22], so values would more readily be interpreted and compared with other literature values. H' was expected to decrease with deterioration of water quality [23]. High values of H' would be representative of more diverse communities (namely good water quality). A community with only one species would have an H' value of 0, and if the species were evenly distributed among the S species then the H' value would be at a maximum. So the H' value allowed us to know not only the number of species but how the abundance of the species was distributed among all the species in the community. [24] suggested a relationship between H' and the pollution status of aquatic ecosystems and classified H' as:
>3.0 = "very good status (clean water)";
1.0-3.0 = "moderate status (moderately polluted)";
<1.0 = "bad status (heavily polluted)".
[25] modified the above mentioned classification by dividing '1.0- 3.0' into two scales as:
2.0-3.0 = "good status (lightly polluted)";
1.0-2.0 = "moderate status (moderately polluted)".
Saprobity index (SaI) [26]
Saprobity index (SaI) is a weighted mean of the individual saprobic value of each species "s" multiplied by their abundance "h" and divided by the total abundance:
where "s" can take values between 1 for oligosaprobic, 2 for β-mesosaprobic, 3 for a-mesosaprobic, 4 for a-meso/polysaprobic and 5 for polysaprobic species according to [27,28]. SaI ranked from 0 to 5 and was characterized as:
<1.8 = "very good status (oligosaprobity)";
1.8-2.3 = "good status (β-mesosaprobity)";
2.3-2.8 = "moderate status (β-α-mesosaprobity)";
2.8-3.3 = "poor status (a-mesosaprobity)";
>3.3 = "bad status (polysaprobity)".
SaI was mainly designed for organic pollution of streams and rivers [7], and has been widely used as an indicator of water quality in reservoirs and rivers to date e.g. [10,29-32].
Trophic diatom index (TDI) [33]
The initial version of the TDI was derived empirically from graphs summarizing percent count vs. dissolved phosphorus concentrations for 86 taxa (genera plus key indicator species [33]). It produced values from 1 (low nutrient concentration) to 5 (very high nutrient concentration). However, to express a clear preference for an index that produces integer values over an extended numerical range, the TDI was therefore modified to 0 (low nutrient concentration) - 100 (very high nutrient concentration). This was achieved as follows:
where:
TDI = trophic diatom index
WMS = weighted mean sensitivity, calculated using sensitivity and indicator values according to [34]:
where:
WMS = weighted mean sensitivity
ai = proportion of all individuals in a sample that belong to species i
n = total number of species in a sample
si = pollution sensitivity (1-5) of species i
vi = indicator values (1-3) of species i
TDI was originally designed for benthic algae, but has been employed for phytoplankton e.g. [4,10,35]. TDI was expected to increase with increasing eutrophication, ranked from 0 to 100, and was classified according to [27] as:
0-25 = "very good status (oligo-eutrophic)";
25-50 = "good status (meso-eutrophic)";
50-75 = "moderate status (eutrophic)";
75-100 = "poor status (hyper-eutrophic)".
Nevertheless, where there was heavy organic pollution, it was difficult to separate the effects of eutrophication from other effects. For this reason, the values of TDI are supplemented by an indication of the percentage pollution tolerant values (%PTV), which is calculated as the sum of values belonging to taxa generally regarded as particularly tolerant to organic pollution. According to [33], %PTV means different organic pollution state:
< 20% = "free of significant organic pollution";
21-40% = "some evidence of organic pollution";
41-60% = "organic pollution likely to contribute significantly to eutrophication of site";
>61% = "site is heavily contaminated with organic pollution".
Q index (QI) [8]
Based on the phytoplankton associations described for lakes [36,37], a new evaluation technique of potamoplankon for the assessment of the ecological status of rivers was proposed by [8]. To achieve an index, each species in the sample must be assigned to the appropriate functional group. Then the relative shares of each functional group are calculated. Relative shares are then multiplied by the factor number and the sum of these scores is the Q index (follow [37]).
where:
QI = Q index
pi = the relative share of the i-th functional group equal to ni/N
ni = the biomass of the i-th group
N = the total biomass
Fi = the factor number (between 0-5).
The method is based on the functional group of algae represented in the potamoplankton and provides a single index number (Q), which has been tested on phytoplankton data of different rivers and proved to be more sensitive than the earlier used index (SaI) [8]. Thereby, the classifications of different functional groups were of great importance for such index and should be gathered from historical studies. Based on the list of the functional groups [36] and its updated version, the evaluation of the 37 functional groups of algae and factor number of each group were provided by [8]. Furthermore, [38] wrote a critical review with updates of the phytoplankton functional classification.
Theoretically, the maximum of QI is 5, while the minimum is 0, and is expected to decrease with decline of the ecological status of rivers. QI values for different water quality classes of proposed river types were summarized in Table 2.
River type
Stream order*
Residence time (day)
QI
Excellent
Good
Moderate
Poor
Bad
Brooks and small streams
1-5
<2
5.00
4.95
4.85
4.75
<4.75
Streams
3-6
2-4
4.95
4.85
4.75
4.50
<4.50
Small rivers (lowland streams)
4-7
4-8
4.75
4.50
4.00
3.50
<3.50
Rivers
6-9
8-12
4.50
4.00
3.50
3.00
<3.00
Large rivers
7-10
12-16
4.00
3.50
3.00
2.50
<2.50
Very large rivers
>10
>16
3.50
3.00
2.50
2.00
<2.00
Table 2: Proposed river types and Q index (QI) values for different water quality classes (modified from [8]).
Phytoplankton index (PhI) [39]
Phytoplankton index (PhI) is also a new approach to assess running waters by phytoplankton introduced by [40] on behalf of the German Working Group on water issues of the Federal States and Federal Government (LAWA) to implement the European Water Framework Directive (WFD, EC 2000). It includes five sub-indices: Assessment value of total pigment (A-valuetotalpigment), Pennales-Index (PeI), Chlorophyte-Index (ChI), Cyanobacteria-Index (CyI) and Trophic index of potamoplankton taxa (TIP). PhI is the mean of single results evaluated by the five sub-indices:
PhI = (A-valuetotalpigment + PeI + ChI + CyI + TIP) / No. of used indices (Equation 6)
The scale of the PhI is in the range of 0.5-5.5:
<1.51 = "very good status";
1.51-2.50 = "good status";
2.51-3.50 = "moderate status";
3.51-4.50 = "low status";
>4.50 = "bad status".
A-valuetotalpigment:
Total pigment is estimated by phytoplankton Chl a values. A-valuetotalpigment is calculated by specific formulas for different catchment types (Table 3).
Catchment type
Formulas from Chl a to A-valuetotalpigment
10.1+20.1
1.8527*Ln(Chl a)-2.7981
15.1+17.1
1.9907*Ln(Chl a)-4.4749
15.2+17.2
1.9907*Ln(Chl a)-4.4749
9.2
1.9907*Ln(Chl a)-4.4749
10.2+20.2
1.8168*Ln(Chl a)-4.6772
Table 3: Formulas used for transformation between Chl a and A-valuetotalpigment in different catchment types (modified from [39]).
PeI, ChI and CyI:
PeI, ChI and CyI are categorized into 5 scales (1-5) based on the percentages of pennales, chlorophyte and cyanobacteria, respectively. The scaling systems have been shown by [39]. For example, a percentage of pennales between 15 and 20 in catchment type 20.1 (large streams with high specific run-off > 10 ls-1km-2) will lead to a PeI value of 2, which means 'good' state.
TIP:
The formula used to calculate TIP is:
where:
TIP = Trophic index of potamoplankton taxa
n = total number of species in a sample
TIi = type-specific index of species i
GWi = weight factor of species i
DWi = proportion of all individuals in sample that belong to species i
GW and TI values of indicator taxa in different catchment types were shown by [39].
Future Directions and Applications
Standardization of sampling methods
The investigation of the phytoplankton community has become an important part of the overall water quality monitoring [41], since reliable quantitative data on species composition are of primary importance for bio-assessment development. The precision obtained in the field may vary greatly due to the differences in sampling methods. There are two main methods for riverine phytoplankton sampling: 1) plankton nets 2) sedimentation protocols [1,42]. The two sampling protocols of phytoplankton were both widely used and had their advantages respectively. For example, the "plankton nets" protocol is labor saving, fast, easy to handle and can capture more rare species, but allows real nannoplankton to pass through its meshes [42,43]. It is thus a preferred method for clean water with low phytoplankton density. In contrast, sedimentation protocol is usually used in water bodies with high phytoplankton density (e.g. [45-49]). The above mentioned methods were both applied in the stream systems (e.g. [4,10,50-54]). Nevertheless, to our knowledge, except for [42], the influence of two sampling protocols on the outcome of bio-assessment in streams has not been investigated systematically yet. By comparison and unification between different sampling methods, phytoplankton data from various areas with different sampling protocols over multiple years could be merged to get a more comprehensive understanding of the ecological status by regional or country-wide assessment.
Relations with Environmental Factors
The response of phytoplankton to surrounding environmental factors has drawn particular attentions of present researches [55] and identification of the main factors controlling phytoplankton in a particular water body is essential for choosing an appropriate management strategy for the maintenance of a desired ecosystem state [56]. Distribution patterns of phytoplankton are strongly correlated with environmental factors [57]. Possible factors may be physical, chemical, hydrological and biotic factors [5,45,54,58- 63]. Unfortunately, there is no general consensus as to which factors regulate phytoplankton community in lotic habitats [64], and contributions of main environmental factors to phytoplankton variations are also unclear [54]. Understanding organism dynamics and resilience of river ecosystems in changing environmental factors (e.g. global changes) will greatly benefit the phytoplankton based bio-assessment.
Bio-indication
The assessment of the ecological status of freshwater ecosystems is a key issue for many international laws such as the European Water Framework Directive (WFD) [65]. Many efforts have been devoted to the development of efficient tools to measure the ecological status of freshwater systems based on fish, macroinvertebrates, macrophytes and diatoms. Generally, all assessment methods can be sorted into three approaches. The first approach is based on the indicator species concept, the second one is based on the diversity of organisms, while third one applies multimetric approaches which are composed of several indices that can reduce information from individuals, population, community and ecosystem. More and more authors prefer the third approach because it integrates, condenses and summarizes biological data, and thus can reflect ecological status in a comprehensive manner [9,66-68]. One example is the multi-metric Index of Biotic Integrity (IBI), originally developed by [69] which is the most common indicator of stream condition in use today. Many assessment methods based on IBI have been developed and used to date in several countries and regions (e.g. [4,23,65,68,70- 76]). Recently, a composite index at regional level named RIEI (Regional Index of Ecological Integrity) for sustainable management of natural resources was proposed by [77], and it is composed by not only "Physical Integrity", "Chemical Integrity", "Biological Integrity" but also by "Beauty", "Biodiversity" and "Ecosystem Health" indices. However, the riverine phytoplankton index of biotic integrity (PIBI) is rarely considered for river 'health' assessment [4]. This is in part because of the former understanding of riverine phytoplankton that algae found in rivers are believed to come from other sources than the rivers themselves - either from lentic waterbodies or the benthos [78]. With the confirmation of riverine phytoplankton, they should be combined with former assessing systems for rivers [54]. The selection of indicator depends on the stressor-type being assessed and the monitoring type. For example, according to [79], diatoms should be considered when the study focus is on nutrient enrichment and at small stream with relatively species-poor fish and macrophyte assemblages. However, in the case of hydromorphological degradation, fish, benthic macroinvertebrates and macrophytes should be considered instead of algae community.
Modeling and predicting riverine phytoplankton
Predicting freshwater phytoplankton dynamics is regarded as one of the important issues in the domain of river ecology and management [80]. The successful prediction by multi-variate processes either for short or long intervals of monitoring could drive the underlying mechanisms between phytoplankton and their environments. From the management decision-making point of view, [80] thought that if an accurate model for phytoplankton dynamics was reliable, then forecasting would be possible with only phytoplankton data instead of monitoring a wide range of limnological changes, which usually has exorbitant costs. Many models, therefore, have been developed and used to simulate freshwater phytoplankton dynamics as well as aquatic insects in lakes and reservoirs, such as ANN (artificial neural networks) based models [5,81-84], PROTECH (Phytoplankton Responses To Environmental Change) [22,85- 88], RIVERSTRAHLER [89,90], NPZ (nutrient/phytoplankton/ zooplankton) [91-93], process-based models [94,95]. However, only very few of them have been used for riverine phytoplankton simulation [5,80,96-98]. Therefore, the future studies should address to the followings: 1) comparing the performances of different models; 2) developing and testing of new comprehensive models that examine the impacts of multiple stressors on riverine phytoplankton. Besides, declining water quality worldwide and increasing progress in predictive potential of ecology and limnology greatly promote the development of the 'Ecohydrology' approach [99-101], which can provide means of integrating landscape hydrology with freshwater biology [102,112] and create an interdisciplinary background (ecological and hydrological) for the assessment and sustainable management of freshwater resources.
References
- Hötzel G, Croome R. A Phytoplankton Methods Manual for Australian Freshwaters. LWRRDC Occasional Paper. 22/99. 1999.
- Domingues R, Galv&aTilde;o H. Phytoplankton and environmental variability in a dam regulated temperate estuary. Hydrobiologia. 2007; 586: 117-134.
- Cabecinha E, Cortes R, Cabral J, Ferreira T, Lourenço M, Pardal M. Multi-scale approach using phytoplankton as a first step towards the definition of the ecological status of reservoirs. Ecological Indicators. 2009; 9: 240-255.
- Wu N, Schmalz B, Fohrer N. Development and testing of a phytoplankton index of biotic integrity (P-IBI) for a German lowland river. Ecological Indicators. 2012; 13: 158-167.
- Wu N, Huang J, Schmalz B, Fohrer N. Modeling daily chlorophyll a dynamics in a German lowland river using artificial neural networks and multiple linear regression approaches. Limnology. 2014; 15: 47-56.
- Cohn F. Über lebende Organismen im Trinkwasser. Z. klein. Medizin 1853; 4: 229-237.
- Dokulil MT. Chapter 9 Algae as ecological bio-indicators. Trace Metals and other Contaminants in the Environment. 2003; 6: 285-327.
- Borics G, Várbíró G, Grigorszky I, Krasznai E, Szabó S, Kiss K. A new evaluation technique of potamo-plankton for the assessment of the ecological status of rivers. Archiv für Hydrobiologie (Supplement). 2007; 161: 465-486.
- Blanco S, Bécares E, Cauchie H, Hoffmann L, Ector L. Comparison of biotic indices for water quality diagnosis in the Duero Basin (Spain). Archiv für Hydrobiologie (Supplement). 2007; 161: 267-286.
- Plenkovic-Moraj A, Gligora M, Kralj K, Perica M. Diatoms in monitoring of Drava River, Croatia. Archiv für Hydrobiologie (Supplement). 2007; 161: 511-525.
- Reavie E, Jicha T, Angradi T, Bolgrien D, Hill B. Algal assemblages for large river monitoring: comparison among biovolume, absolute and relative abundance metrics. Ecological Indicators. 2010; 10: 167-177.
- Pfister L, McDonnell JJ, Wrede S, Hlúbiková D, Matgen P, Fenicia F, et al. The rivers are alive: on the potential for diatoms as a tracer of water source and hydrological connectivity. Hydrological Processes. 2009; 23: 2841-2845.
- Martínez-Carreras N, Frentress J, Iffly JF, McDonnell J, Hlúbiková D, Ector L, et al. Using diatoms for determining the hydrological connectivity between upland, riparian and aquatic zones: application to the Weierbach catchment (Luxembourg). Geophysical Research Abstracts. 2011; 13.
- Klaus J, Wetzel CE, Martínez-Carreras N, Ector L, Pfister L. Diatoms as a fingerprint of sub-catchment contributions to meso-scale catchment runoff. Geophysical Research Abstracts. 2014; 16: EGU2014-11124-1.
- Wetzel CE, Klaus J, Martínez-Carreras N, Ector L, Pfister L. Hydrogeological and landscape controls on terrestrial diatoms input to the stream network during rainfall-runoff events. Geophysical Research Abstracts 2014; 16: EGU2014-10604.
- Hillebrand H, Dürselen C, Kirschtel D, Pollingher U, Zohary T. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology. 1999; 35: 403-424.
- Kononen K, Forsskähl M, Huttunen M, Sandell M, Viljamaa H. Practical problems encountered in phytoplankton cell volume using the BMB recommendation in the Gulf of Finland. Limnologica. 1984; 15: 605-614.
- Sun J, Liu D. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research. 2003; 25: 1331-1346.
- Vadrucci M, Cabrini M, Basset A. Biovolume determination of phytoplankton guilds in transitional water ecosystems of Mediterranean Ecoregion. Transitional Waters Bulletin. 2007; 2: 83-102.
- Padisák J, Adrian R. Biovolumen. Tümpling v. W, Friedrich G, editors. In: Methoden der Biologischen Wasseruntersuchung 2. Biologische Gewässeruntersuchung, Chapter 5.1. Gustav Fischer Verlag, Jena. 1999; 334-367.
- Shannon CE, Weaver W. The mathematical theory of communication. University of Illinois Press, Urbana, Illinois. 1949.
- Karydis M, Tsirtsis G. Ecological indices: a biometric approach for assessing eutrophication levels in the marine environment. Science of the Total Environment. 1996; 186: 209-219.
- Wang Y, Stevenson R, Metzmeier L. Development and evaluation of a diatom-based index of biotic integrity for the Interior Plateau Ecoregion, USA. Journal of the North American Benthological Society. 2005; 24: 990-1008.
- Wilhm J, Dorris T. Species diversity of benthic macroinvertebrates in a stream receiving domestic and oil refinery effluents. American Midland Naturalist. 1966; 76: 427-449.
- Staub R, Appling J, Hofsteiler A, Hess I. The effect of industrial wastes of Memphis and Shelby country on primary plankton producers. Bioscience. 1970; 20: 905-912.
- Pantle R, Buck H. Die Biologische Überwachung der Gewässer und die Darstellung der Ergebnisse. Gas u. Wasserfach. 1955; 96: 604.
- van Dam H, Mertens A, Sinkeldam J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Netherlands Journal of Aquatic Ecology. 1994; 28: 117-133.
- Porter SD. Algal attributes: an autecological classification of algal taxa collected by the National Water-Quality Assessment Program. U.S. Geological Survey Data Series 329. 2008.
- Kaatra K. Saprobiological evaluation of the Silvola reservoir. Acta Hydrochim. Hydrobiol. 1978; 6: 321-328.
- Wu J. Phytoplankton as bioindicator for water quality in Taipei. Botanical Bulletin of Academia Sinica. 1984; 25: 205-214.
- Korneva L, Mineeva N. Phytoplankton composition and pigment concentrations as indicators of water quality in the Rybinsk reservoir. Hydrobiologia. 1996; 322: 255-259.
- Rankovic B, Simic S, Bogdanovic D. Phytoplankton as indicator of water quality of lakes Bubanj and Šumarice during autumn. Kragujevac J. Sci. 2006; 28: 107-114.
- Kelly M, Whitton B. The trophic diatom index: a new index for monitoring eutrophication in rivers. Journal of Applied Phycology. 1995; 7: 433-444.
- Kelly M. Use of the trophic diatom index to monitor eutrophication in rivers. Water Research. 1998; 32: 236-242.
- Irvine K, Murphy T. Assessment of eutrophication and phytoplankton community impairment in the Buffalo River Area of Concern. Journal of Great Lakes Research. 2009; 35: 83-93.
- Reynolds C, Huszar V, Kruk C, Naselli-Flores L, Melo S. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research. 2002; 24: 417-428.
- Padisák J, Grigorszky I, Borics G, Soróczki-Pintér É. Use of phytoplankton assemblages for monitoring ecological status of lakes within the Water Framework Directive: the assemblages index. Hydrobiologia. 2005; 553: 1-14.
- Mischke U, Behrendt H. Handbuch zum Bewertungsverfahren von Flieβgewässern mittels Phytoplankton zur Umsetzung der EU-Wasserrahmenrichtlinie in Deutschland. WeiβenseeVerlag, Berlin. ISBN 978-3-89998-105-6. 2007; pp 88 (in German).
- Mischke U, Behrendt H, Köhler J, Opitz D. Report: Entwicklung eines Bewertungsverfahrens für Flieβgewässer mittels Phytoplankton zur Umsetzung der EU-Wasserrahmenrichtlinie. LAWA 03.05 IGB Berlin. 2005; pp 99 (in German).
- Majaneva M, Autio R, Huttunen M, Kuosa H, Kuparinen J. Phytoplankton monitoring: the effect of sampling methods used during different stratification and bloom conditions in the Baltic Sea. Boreal Environment Research. 2009; 14: 313-322.
- .Wu N, Schmalz B, Fohrer N. A comparison of phytoplankton assemblages generated by two sampling protocols in a lowland catchment, Germany. Annales de Limnologie / International Journal of Limnology. 2011a; 47: 313-323.
- Kraatz W. A comparison of plankton counts from the trap-net and water bottle centrifuge techniques. The Ohio Journal of Science. 1940; 40: 151-161.
- #
- Tangen K. Nets. Sournia A. editors. In: Phytoplankton manual, UNESCO, Norwich. 1978; pp 50.
- Ha K, Kim H, Joo G. The phytoplankton succession in the lower part of hypertrophic Nakdong River (Mulgum), South Korea. Hydrobiologia. 1998; 369/370: 217-227.
- Köhler J, Bahnwart M, Ockenfeld K. Growth and loss processes of riverine phytoplankton in relation to water depth. International Review of Hydrobiology. 2002; 87: 241-254.
- Sabater S, Artigas J, Durán C, Pardos M, Romaní AM, Tornés E, et al. Longitudinal development of chlorophyll and phytoplankton assemblages in a regulated large river (the Ebro River). Sci Total Environ. 2008; 404: 196-206.
- Friedrich G, Pohlmann M. Long-term plankton studies at the lower Rhine / Germany. Limnologica. 2009; 39: 14-39.
- Xu Y, Cai Q, Ye L, Zhou S, Han X. Spring diatom blooming phases in a representative eutrophic bay of the Three-Gorges Reservoir, China. Journal of Freshwater Ecology. 2009; 24: 191-198.
- Trifonova I, Pavlova O. Assessment of the Trophic State of Lake Ladoga Tributaries and the Neva River by phytoplankton. Water Resources. 2004; 31: 679-688.
- Trifonova I, Pavlova O, Rusanov A. Phytoplankton as an indicator of water quality in the rivers of the Lake Ladoga basin and its relation to environmental factors. Archiv für Hydrobiologie (Supplement). 2007; 161: 527-549.
- Wu NC, Tang T, Zhou SC, Fu XC, Jiang WX, Li FQ, et al. [Influence of cascaded exploitation of small hydropower on phytoplankton in Xiangxi River]. Ying Yong Sheng Tai Xue Bao. 2007; 18: 1091-1096.
- Centis B, Tolotti M, Salmaso N. Structure of the diatom community of the River Adige (North-Eastern Italy) along a hydrological gradient. Hydrobiologia. 2010; 639: 37-42.
- Wu N, Schmalz B, Fohrer N. Distribution of phytoplankton in a German lowland river in relation to environmental factors. Journal of Plankton Research. 2011b; 47: 313-323.
- Buric Z, Cetinic I, Vilicic D, Mihalic K, Caric M, Olujic G. Spatial and temporal distribution of phytoplankton in a highly stratified estuary (Zrmanja, Adriatic Sea). Marine Ecology. 2007; 28: 169-177.
- Peretyatko A, Teissier S, Symoens J, Triest L. Phytoplankton biomass and environmental factors over a gradient of clear to turbid peri-urban ponds. Aquatic Conservation: Marine and Freshwater Ecosystems. 2007; 17: 584-601.
- Lepistö L, Holopainen A, Vuoristo H. Type-specific and indicator taxa of phytoplankton as a quality criterion for assessing the ecological status of Finnish boreal lakes. Limnologica. 2004; 34: 236-248.
- Reynolds C, Padisák J, Sommer U. Intermediate disturbance in the ecology of phytoplankton and the maintenance of species diversity: A synthesis. Hydrobiologia. 1993; 249: 183-188.
- Kiss K, Ács É, Kovács A. Ecological observation on Skeletonema potamus (Weber) Hasle in the River Danube, near Budapest (1991-92, daily investigations). Hydrobiologia. 1994; 289: 163-170.
- Skidmore R, Maberly S, Whitton B. Patterns of spatial and temporal variation in phytoplankton chlorophyll a in the River Trent and its tributaries. Science of Total Environment. 1998; 210-211: 357-365.
- Torremorell A, Llames M, Pérez G, Escaray R, Bustingorry J, Zagarese H. Annual patterns of phytoplankton density and primary production in a large, shallow lake: the central role of light. Freshwater Biology. 2009; 54: 437-449.
- Mutshinda CM, Finkel ZV, Irwin AJ. Which environmental factors control phytoplankton populations? A Bayesian variable selection approach. Ecological Modelling. 2013; 269: 1-8.
- Tang T, Wu N, Li F, Fu X, Cai Q. Disentangling the roles of spatial and environmental variables in shaping benthic algal assemblages in rivers of central and northern China. Aquatic Ecology. 2013; 47: 453-466.
- Basu B, Pick F. Longitudinal and seasonal development of planktonic chlorophyll a in the Rideau River, Ontario. Canadian Journal of Fisheries and Aquatic Sciences. 1995; 52: 804-815.
- Hermoso V, Clavero M, Blanco-Garrido F, Prenda J. Assessing the ecological status in species-poor systems: A fish-based index for Mediterranean Rivers (Guadiana River, SW Spain). Ecological Indicators 2010; 10: 1152-1161.
- Lydy M, Crawford C, Frey J. A comparison of selected diversity, similarity, and biotic indices for detecting changes in benthic-invertebrate community structure and stream quality. Archives of Environmental Contamination and Toxicology. 2000; 39: 469-479.
- Triest L, Kaur P, Heylen S, De Pauw N. Comparative monitoring of diatoms, macroinvertebrates and macrophytes in the Woluwe River (Brussels, Belgium). Aquatic Ecology. 2001; 35: 183-194.
- Tang T, Cai Q, Liu J. Using epilithic diatom communities to assess ecological condition of Xiangxi river system. Environ Monit Assess. 2006; 112: 347-361.
- Karr J. Assessment of biotic integrity using fish communities. Fisheries. 1981; 6: 21-27.
- Prygiel J, Coste M. The assessement of water quality in the Artois-Picardie water basin (France) by use of diatom indices. Hydrobiologia. 1993; 269/270: 343-349.
- Hill BH, Herlihy AT, Kaufmann PR, DeCelles SJ, Borgh MAV. Assessment of streams of the eastern United States using a periphyton index of biotic integrity. Ecological Indicators. 2003; 2: 325-338.
- Mattsson B, Cooper R. Louisiana waterthrushes (Seiurus motacilla) and habitat assessments as cost-effective indicators of instream biotic integrity. Freshwater Biology. 2006; 51: 1941-1958.
- Rothrock P, Simon T, Stewart P. Development, calibration, and validation of a littoral zone plant index of biotic integrity (PIBI) for lacustrine wetlands. Ecological Indicators. 2008; 8: 79-88.
- Bae D, Kumar H, Han J, Kim J, Kim K, Kwon Y, et al. Integrative ecological health assessment of an acid mine stream and in situ pilot tests for wastewater treatments. Ecological Engineering 2010; 36: 653-663.
- Li F, Cai Q, Ye L. Developing a Benthic Index of Biological Integrity and some relationships to environmental factors in the subtropical Xiangxi River, China. International Review of Hydrobiology. 2010; 95: 171-189.
- Zalack J, Smucker N, Vis M. Development of a diatom index of biotic integrity for acid mine drainage impacted streams. Ecological Indicators. 2010; 10: 287-295.
- Reza M, Abdullah S. Regional Index of Ecological Integrity: A need for sustainable management of natural resources. Ecological Indicators. 2011; 11: 220-229.
- Reynolds C. Potamoplankton: paradigms, paradoxes and prognoses. Round FE, editors. In: Algae and the aquatic environment. Biopress, Bristol. 1988; 285-311.
- Hering D, Johnson RK, Kramm S, Schmutz S, Szoszkiewicz K, Verdonschot PFM. Assessment of European streams with diatoms, macrophytes, macroinvertebrates and fish: a comparative metric-based analysis of organism response to stress. Freshwater Biology. 2006; 51: 1757-1785.
- Jeong K, Kim D, Jung J, Kim M, Joo G. Non-linear autoregressive modelling by Temporal Recurrent Neural Networks for the prediction of freshwater phytoplankton dynamics. Ecological Modelling. 2008; 211: 292-300.
- Schleiter I, Borchardt D, Wagner R, Dapper T, Schmidt K, Schmidt H, et al. Modelling water quality, bioindication and population dynamics in lotic ecosystems using neural networks. Ecological Modelling. 1999; 120: 271-286.
- Obach M, Wagner R, Werner H, Schmidt H. Modelling population dynamics of aquatic insects with artificial neural networks. Ecological Modelling. 2001; 146: 207-217.
- Dedecker A, Goethals P, D'heygere T, Gevrey M, Lek S, De Pauw N. Application of artificial neural network models to analyse the relationships between Gammarus pulex L. (Crustacea, Amphipoda) and river characteristics. Environmental Monitoring and Assessment. 2005; 111: 223-241.
- Goethals P, Dedecker A, Gabriels W, Lek S, De Pauw N. Applications of artificial neural networks predicting macroinvertebrates in freshwaters. Aquatic Ecology. 2007; 41: 491-508.
- Elliott J, Irish A, Reynolds C, Tett P. Modelling freshwater phytoplankton communities: an exercise in validation. Ecological Modelling. 2000; 128: 19-26.
- Elliott J, Persson I, Thackeray S, Blenckner T. Phytoplankton modelling of Lake Erken, Sweden by linking the models PROBE and PROTECH. Ecological Modelling. 2007; 202: 421-426.
- Elliott J, Irish A, Reynolds C. Modelling phytoplankton dynamics in fresh waters: affirmation of the PROTECH approach to simulation. Freshwater Reviews. 2010; 3: 75-96.
- Reynolds C, Irish A, Elliott J. The ecological basis for simulating phytoplankton responses to environmental change (PROTECH). Ecological Modelling. 2001; 140: 271-291.
- Billen G, Garnier J, Hanset P. Modelling phytoplankton development in whole drainage networks: the RIVERSTRAHLER Model applied to the Seine river system. Hydrobiologia. 1994; 289: 119-137.
- Even S, Thouvenin B, Bacq N, Billen G, Garnier G, Guezennec L, et al. An integrated modelling approach to forecast the impact of human pressure in the Seine estuary. Hydrobiologia. 2007; 588: 13-29.
- Franks P, Wroblewski J, Flierl G. Behaviour of a simple plankton model with food-level acclimation by herbivores. Marine Biology. 1986; 91: 121-129.
- Baird M, Emsley S. Towards a mechanistic model of plankton population dynamics. Journal of Plankton Research 1999; 21: 85-126.
- Baird M, Oke P, Suthers I, Middleton J. A plankton population model with biomechanical descriptions of biological processes in an idealised 2D ocean basin. Journal of Marine Systems. 2004; 50: 199-222.
- Huang JC, Gao JF, Mooij WM, Hörmann G, Fohrer N. A comparison of three approaches to predict phytoplankton biomass in Gonghu bay of Lake Taihu. J Environmental Informatics. 2014a (in press).
- Huang JC, Gao JF, Mooij WM, Hörmann G, Fohrer N. Modeling the effects of environmental variables on short-term spatial changes in phytoplankton biomass in a large shallow lake, Lake Taihu. Environment al Earth Science. 2014b.
- Thebault J, Qotbi A. A model of phytoplankton development in the Lot river (France). simulations of scenarios. Water Research. 1999; 33: 1065-1079.
- Everbecq E, Gosselain V, Viroux L, Descy JP. Potamon: a dynamic model for predicting phytoplankton composition and biomass in lowland rivers. Water Res. 2001; 35: 901-912.
- Jeong K, Joo G, Kim H, Ha K, Recknagel F. Prediction and elucidation of phytoplankton dynamics in the Nakdong River (Korea) by means of a recurrent artificial neural network. Ecological Modelling. 2001; 146: 115-129.
- de Beauregard A, Torres G, Malaisse F. Ecohydrology: a new paradigm for bioengineers. Biotechnology, Agronomy, Society and Environment. 2002; 6: 17-27.
- Zalewski M. Ecohydrology - framework for implementation of ecological biotechnologies in integrated water resource management (IWRM). Folia Geographica Series Geographica - Physica. 2008; 39: 53-62.
- Zalewski M. Ecohydrology: process-oriented thinking towards sustainable river basins. Ecohydrology & Hydrobiology. 2013; 13: 97-103.
- Bonell M. Ecohydrology-a completely new idea? Hydrological Sciences Journal. 2002; 47: 809-810.
- APHA. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, New York. 1992.
- U.S. Environmental Protection Agency (U.S. EPA). Lake Michigan mass balance, methods compendium: LMMB 065 (ESS Method 340.2). Wisconsin State Lab of Hygiene, Madison. 1997; 3.
- Matthews RA, Kondratieff PF, Buikema AL Jr. A field verification of the use of the autotrophic index in monitoring stress effects. Bull Environ Contam Toxicol. 1980; 25: 226-233.
- Menhinick E. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology. 1964; 45: 859-861.
- Pielou E. The measurement of diversity in different types of biological collections. Journal of Theoretical Biology. 1966; 13: 131-144.
- Sheldon A. Equitability indices: dependence on species count. Ecology. 1969; 50: 466-467.
- Ludwig AJ, Reynolds JF. Statistical Ecology: A Primer on Methods and Computing. John Wiley and Sons, New York. 1988.
- Camargo JA. Revisiting the relation between species diversity and information theory. Acta Biotheor. 2008; 56: 275-283.
- Margalef R. Information theory in ecology. General Systems. 1958; 3: 36-71.
- Tschmelak J, Proll G, Riedt J, Kaiser J, Kraemmer P, Bárzaga L, et al. Automated Water Analyser Computer Supported System (AWACSS) Part II: Intelligent, remote-controlled, cost-effective, on-line, water-monitoring measurement system. Biosens Bioelectron. 2005; 20: 1509-1519.