
Research Article
Austin J Hydrol. 2015;2(1): 1013.
Water Quality of the Vadose and Saturated Zone in Part of the Sedimentary Aquifer System of Kopais Basincentral Greece
Konstantinou V¹*, Arampatzis G², Panagopoulos A¹, Tziritis E¹ and Voudouris K²
¹Soil and Water Resources Institute, Hellenic Agricultural Organization, Greece
²Department of Geology, Aristotle University, Greece
*Corresponding author: Konstantinou V, Soil and Water Resources Institute, Hellenic Agricultural Organization, 57400 Sindos, Greece
Received: April 27, 2015; Accepted: May 29, 2015; Published: June 01, 2015
Abstract
This paper aims at presenting the study of groundwater quality and more specifically of the aquifer and the leachates of sub-soil at the central part of Viotikos Kifisos river basin-central Greece. Quality characterization of the aquifer system was based on the principles set in the Water Framework Directive-WFD (2000/60/EC), adopting as standards the maximum admissible concentrations for water intended for human consumption. Results were based on the analysis of hydrochemical determinations carried out in 194 water samples collected from the vadose zone (through a prototype sampling system) and the shallow wells of the saturated zone, over the period 2009-2011. Spatio-temporal study of water quality characteristics was performed and the hydrochemical character of water samples was assessed using the expanded Durov diagram. As suggested by the analysis of the hydrochemical data, the bedrock of the basin that is formed of carbonate rocks and a schist-chert formation with ultrabasic blocks (mélange), control the dominant hydrochemical character of the analyzed water samples. Hence a strong calcium bicarbonate character prevails, whilst the existence of iron, manganese and nickel is characteristic and attributed to weathering of the lateritic horizons and supplementary to the weathering of ultrabasic blocks. Intensive agriculture that is the dominant activity in the wider region is clearly depicted especially in the vadose zone leachates, in the form of elevated ammonium concentrations that often exceed the maximum admissible concentration for water intended for potable use. In addition, nitrate concentrations in the saturated zone of the sedimentary aquifer system exceed the respective threshold in several cases.
Keywords: Groundwater; Hydrogeochemistry; Vadose zone; Water quality; Kopais basin
Introduction
The leachates of the vadose (unsaturated) zone provide a representative sample, in order to assess the potential impact of anthropogenic activities to the environment and especially to the groundwater aquifer systems. Leachates are formed when water having uptake solutes infiltrates from the soil surface through the vadose zone, thus potentially reaching the saturated zone of an aquifer system. The vadose zone is not a water body in strict hydrogeological terms, however, the quality characteristics of the leachates, when moving through the vadose zone deeper than the bulk of the rooting system, provide important information about the potential pollution load, which could reach groundwater resources.
The study area (Figure 1) is located in the Prefecture of Viotia, in Central Greece and occupies the central part of the Viotikos Kifisos river basin. Intensive agricultural activities combined with the geological background, set the area notably vulnerable to pollution from agrochemicals, as can be revealed from both current measurements and analyses within previous research projects and development studies [1]. Moreover, the study area is of significant importance since the runoff of the basin end up through Kifisos River into Yliki Lake. The karstic aquifer system of the basin covers the potable water demands of the wider area, supports the locally developing terrestrial ecosystems and hosts strategic reservoirs of the Attica basin [2].
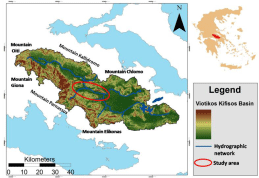
Figure 1: Viotikos Kifisos river basin, showing the study area [4].
The study area is bounded from Mt Akontio (502 m) to the north, to Mt Thourio (511 m) to the south, and the villages of Mavroneri and Orchomenos to the west and east, respectively. Annual and intensive cultivations set agriculture as the dominant anthropogenic activity in the area. The average altitude is 110 m and the average annual rainfall is 600 mm. The main climatic characteristic of the Viotikos Kifisos region is the alternation from a wet and cold season which lasts from October to early May, to a dry and hot one which runs from mid- May to September. The two-season distinction has been based on the criteria of rainfall and temperature temporal distribution. The wider region constitutes a receptor of large amounts of surface water [3], due to the geomorphology, geological setting, amount of rainfall and amount of snow cover in the mountainous area bordering the basin.
The geological setting of the basin consists of formations that belong to Parnassos - Giona (External Hellenides) and to Sub-Pelagonian (Internal Hellenides) tectonic zones, and to the geotectonical unit of Viotia. Geomorphologically, the study area belongs to Kopais basin and is covered by Quaternary fluvial deposits, consisting of clays, sands, gravels, pebbles and cobbles. The Alpine rocks composing the bedrock of the basin are cropping out in the surrounding area and include limestones, dolomites, schist-chert conurbation with ophiolites and formations of flysch [3-5].
Hydrogeologically, the study area belongs to the mid and lower route of Viotikos Kifisos river basin, before entering into the main Kopais basin. Developed within the coarse Quaternary deposits of the study area, the shallow alluvial aquifer, is characterized by moderate to low permeability values (K=10-4-10-6 m/s). The dominant groundwater flow direction in the particular part of the shallow aquifer is from SW to NE. Depth to water table decreases from south to north and from NW to SE and ranges from 1-7.5 m and 0.5-5.5 m, over the period April and July 2009 respectively. The role of the aquifer, however, is particularly important not only in covering part of local irrigation needs but most importantly in acting as a buffer protection zone to water resources of the underlying high potential karstic system.
Materials and Methods
Data collection and analyses
Chemical analyses to 194 water samples from both the vadose zone and the sedimentary aquifer system were undertaken. Systematic monitoring took place from 2009 to 2011 and has been conducted by the Land Reclamation Institute of the Hellenic Agricultural Organization “DEMETER”, in the framework of the project “Strategic plan for the adaptation and application of the principles for the sustainable use of pesticides in a vulnerable ecosystem “- LIFE07 ENV/GR/0000266 EcoPest. A prototype sampling system was installed at selected points of the study area in order to collect leachates from the vadose zone, immediately under the bulk rooting zone of the dominant cultivations [6]. Water sampling from the alluvial aquifer was performed through a monitoring network of shallow wells. Hydrochemical analyses were undertaken in the accredited laboratory of the Land Reclamation Institute (ELOT EN ISO/IEC 17025:2005). Figure 2 shows the spatial distribution of the monitoring network. Depending on their type, monitoring sites are denoted by “W” (shallow wells) for the saturated zone and by “V” for the vadose zone.
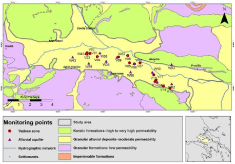
Figure 2: Monitoring network.
Hydrochemical data processing
An analysis of median values of parameters and further graphical representation of those from the vadose and saturated zone, were carried out. Graphical representation was performed by creating expanded Durov diagram, on the base of which hydrochemical types of the samples were determined and hydrochemical processes prevailing in the aquifer system were identified.
The results of the above analysis were used for the assessment and characterization of the chemical quality state of the groundwater system, which was based on the methodology adopted in the elaboration of the national water resources management plans for the river basins of Greece [7].
Firstly, threshold values were set for the considered parameters, adopting as such the Maximum Admissible Concentrations (MAC) for water intended for human consumption, assuming this as the most sensitive water use and given the fact that water resources in the study area partly used for domestic supplies [8,9]. In line with the principles and concepts of the WFD, these limits are accepted as thresholds for cases where natural background concentrations (Natural Background Level - NBL) of a given parameter are lower. In cases where NBL’s are exceeding MAC’s, due to natural causes, the values were adjusted accordingly to meet the physical conditions prevailing in groundwater systems. This is imperative, since, according to the Directives [10,11], high concentrations in excess of assigned maximum acceptable limits for a particular use, which do not occur due to anthropogenic causes, do not consist a degradation factor for the chemical quality of water resources, from the environmental perspective.
Next step was to calculate the median annual values of the parameters in every sampling point and to compare them to the corresponding threshold values. These values provide the measure of the chemical quality state of the sedimentary aquifer. Additionally, the mean annual values of each parameter were compared to the third quartile (Q3-75th %) of the corresponding threshold value, which was introduced as a “trigger value’ or as a starting point for implementing measures of trend reversal, or at least protective measures against further deterioration. If the median concentration value exceeds the trigger value for any of the examined parameters, then the groundwater at the particular monitoring point is characterized to be “at risk’. Depending on their characterization as determined in accordance to aforementioned methodology in “good’, “at risk’ or “poor’, monitoring points are denoted by green, orange and red color codes respectively.
Subsequently, the magnitude of exceedances (i.e. the percentage of monitoring points for which the median annual values of parameters is exceeding the threshold) was calculated. The groundwater system is characterized to be in “poor chemical state’ when more than 20% of the monitoring points is exceeding the threshold (i.e. are characterized as being in poor state). In every other case, it is considered to be in “good chemical state’.
Finally, the chemical state of the aquifer system is represented by compiling a thematic map using color codes. Groundwater systems having been assessed in good state are denoted by green color, while those in poor state by red [7].
Results and Discussion
Table 1 presents the descriptive statistics (minimum, median, maximum values for each parameter) of the hydrochemical data over the examined period 2009-2011.
Parameters
Units
Vadose zone
Alluvial Aquifer
MIN
MAX
MED
MIN
MAX
MED
pH (in situ)
-
6.7
8.1
7.2
6.6
7.9
7.3
E.C. (in situ )
μS/cm
292
1864
778
588
1294
973
K
mg/L
0.4
68.0
2.6
0.2
20.1
1.2
Na
mg/L
3
74
17
11
69
24
Ca
mg/L
39
202
108
42
207
108
Mg
mg/L
3
166
23
21
97
38
Cl
mg/L
4
102
23
9
104
39
CO3
mg/L
0
60
24
0
48
24
HCO3
mg/L
183
769
378
281
586
406
SO4
mg/L
1
442
45
19
294
103
NO3
mg/L
0
285
28
1
143
59
NH4
mg/L
0.1
19.7
0.8
0.1
10.3
0.4
Fe
μg/L
32
11355
192
4
2024
101
Cu
μg/L
0
150
10
0
32
4
Zn
mg/L
0
0.59
0.07
0.01
0.79
0.07
Mn
μg/L
1
5840
25
0
1224
10
Ni
μg/L
1
196
10
0
269
6
Pb
μg/L
<2.5
26.7
<2.5
<2.5
11.3
<2.5
Cd
μg/L
<0.15
1.3
<0.15
<0.15
0.4
<0.15
Table 1: Descriptive statistics of hydrochemical data for the period 2009-2011.
Concentration of calcium (Ca2+) and bicarbonate (HCO3 -) ions indicate that the karstic substrate is dominant in the region, setting a distinct control on the conditions shaping the hydrochemical type of groundwater. This is expected considering the geological setting of the wider region.
The wide range of electrical conductivity in the vadose zone could be attributed to the enriched salts concentration (e.g. hardpans and salt crusts) in the top soil cover at some of the sampling points. In turn, it could be argued that this reflects inadequate leaching of the cropland due to the adopted irrigation practices that leads progressively to accumulation of salts. The sedimentary aquifer system presents less variance than the vadose zone, with an elevated median value at 973μS/cm. However, this value is neither alarming nor constraining for water use.
Heavy metals exhibit in general low concentrations with the exception of iron (Fe), nickel (Ni) and manganese (Mn), which are attributed to the regional lithology [12]. Observed high concentrations of Fe, Ni and Mn can be the result of leaching of Fe - Mn oxides in the soil, or the influence of the ultramafic blocks of schist-chert formation and, supplementary, by the weathering of the lateritic horizons. Similar conditions have been reported in the wider region [12,13].
Median concentrations of nitrates in the vadose zone are low (28 mg/L) but with wide variance from 0 to 285 mg/L, while the same value for ammonium (NH4 +) is 0.8 mg/L, higher than the potable limit (0.5 mg/L). It is worth mentioning that concentrations exceeding 0.5 mg/L (maximum admissible concentration) ranging from 0.1 mg/L to 19.7 mg/L have been documented in all sites of vadose zone. Concentration levels of aforementioned nitrogen compounds along with the wide range of variance in the study area indicate intense agricultural activity and in particular the use of nitrogen fertilizers.
As for the alluvial aquifer system, the concentration of nitrates have a median value of 59 mg/L, which is higher than the potable limit (50 mg/L) and also documents the impact of agricultural activities. For ammonium ions, lower concentrations compared to the vadose zone are observed, with a median value of 0.4 mg/L. This fact can be attributed to the progressive oxidation of nitrogen compounds within the alluvial aquifer in open system conditions under sufficient oxygen supply. Thus, the ammonium ions are gradually oxidized moving from through the vadose zone to the saturated zone, in accordance to the chemical reaction [14]:
2O2 +??4 + → ??3 - + 2?+ +?2?
Figure 3 presents the median values of ammonium and nitrate ions for each sampling point and their limits plotted in the expanded Durov diagram.
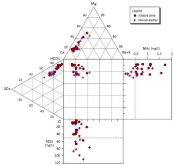
Figure 3: Expanded Durov plot.
Equally remarkable is the presence of sulfate ions that could be related with the applied fertilizers, such as ammonium sulfate, which probably comes from the use of fertilizers. The median concentrations of sodium (Na+), magnesium (Mg2+) and chloride (Cl-) are within the maximum admissible concentrations.
Distribution of the nitrogen compounds in the vadose zone and in the alluvial aquifer is illustrated in the expanded Durov diagram (Figure 3). It is also observed that the Ca-HCO3 character of the aquifer is not as strong as that of the vadose zone, due to the process of intermixing.
The parameters Mn, Ni and Fe have concentrations higher than
the MAC’s for water intended for human consumption [8], but as
mentioned above, this is due to natural causes. Therefore, values
representative of the natural background of the area should be used
for each of the above ions in the chemical quality characterization
process. Alternative methods are proposed for the derivation of
such values. According to Matchullat et al. [15] the iterative-2σ
methodology provides the most representative results, depending on
the nature of the initial statistical population. The two fundamental
requirements imposed are the normal or log-normal distribution of
the statistical population and the sufficient number of samples (n>50)
[16]. According to this methodology NBL is the concentration derived
by the mean+2σ value of 97.5% on the population that is reduced by
the mean+-2σ values (0-2.5% and 97.5-100%) criterion. An alternative
approach proposed in the framework of the implementation of the
Groundwater Daughter Directive [11], suggests the use of the 90th
or the 97.7
Parameters
Natural background values
Mn (μg/L)
1125
Ni (μg/L)
64
Fe (μg/L)
715
Table 2: Adopted natural background levels for Mn, Ni and Fe.
Consequently, in most sampling points of the sedimentary aquifer system (8 out of 12) the chemical state of the water is characterized as “poor’, mainly because of the high concentrations of nitrates and ammonium. These data provide clear evidence of the irrational use of nitrogen fertilizers in the region, which given the limited thickness of the vadose zone, the locally favorable soil conditions for infiltration of pollutants , and the high intrinsic vulnerability of the system as already discussed, have caused nitrate pollution of the sedimentary aquifer.
Table 2 presents the median concentration values of parameters compared with the limits for human consumption and with the calculated natural background levels for each sampling point of the sedimentary aquifer system over the period 2009-2011.
In conclusion, as presented in Table 3, the chemical state of the sedimentary aquifer system is characterized in “poor condition’, since the concentrations of ??3 - and ??4 + are higher than the threshold values in more than 20% of the sampling points, based on the “most sensitive’ limits for human consumption. Figure 4 presents the chemical state of the sedimentary groundwater body in accordance with Directive 2000/60 EC.
Sample points
pH
E.C. μS/cm
K mg/L
Na mg/L
Cl mg/L
SO4 mg/L
NO3 mg/L
NH4 mg/L
Fe μg/L
Cu μg/L
Mn μg/L
Ni μg/L
Pb μg/L
Cd μg/L
Chemical state
W02
7.3
1167
2
32
51
219
110
0.45
67
3.63
13
7
<2.5
<0.15
Poor
W08
7.4
1058
1
61
36
155
76
0.72
100
6.35
55
9
<2.5
<0.15
Poor
W10
7.3
850
3
23
27
60
56
1.22
158
1.82
61
9
<2.5
<0.15
Poor
W16
7.3
698
0.3
17
16
47
12
0.51
117
6.38
17
5
<2.5
<0.15
Poor
W23
7.3
742
6
16
28
75
10
0.37
152
5
939
18
<2.5
<0.15
At risk
W32
7.3
762
1
20
22
61
22
0.44
134
4.1
138
4
<2.5
<0.15
At risk
W34
7.0
1037
1
23
39
123
80
0.22
32.5
3.4
2
2
<2.5
<0.15
Poor
W36
7.0
1021
1
23
53
147
60
0.32
112
4.17
10
6
<2.5
<0.15
Poor
W39
7.1
746
1
21
35
74
29
0.11
79
3.15
1
3
3.5
<0.15
Good
W43
7.2
811
1
36
47
84
45
0.19
52
4.75
1
44
<2.5
<0.15
At risk
W45
7.3
1069
2
31
55
124
99
0.55
53
2.72
6
3
<2.5
<0.15
Poor
W56
6.9
1044
2
24
50
152
83
0.14
221
12.2
9
9
<2.5
<0.15
Poor
MAC
6.5-9.5
2500
12
200
250
250
50
0.50
715
2000
1125
64
10
5
0.75 MAC
1875
9
150
187.5
187.5
37.5
0.375
536
1500
844
48
7.5
3.75
Table 3: Characterization of the chemical quality of each sample point based on the adopted methodology.
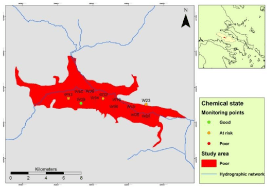
Figure 4: Chemical state of sedimentary groundwater body in accordance
with Directive 2000/60 EC.
Conclusions
As indicated by the analysis of the water samples from the sedimentary aquifer system and the vadose zone, the strong calcium bicarbonate character and high concentrations of certain heavy metals reflect the strong influence of the geological structure in groundwater chemistry. The chemical state of the leachates of the vadose zone clearly indicates that agricultural activities (mainly use of nitrogen fertilizers), provide a pollution load to the area. This is also reflected in the water quality of the sedimentary aquifer system. As water infiltrates, provides the aquifer with nutrients causing nitrate pollution. This diffuse type of pollution occurs in the alluvial aquifer due to cumulative nitrate concentration, which in some cases reaches levels that are prohibitive for human consumption. Based on the application of Directive 2000/60EC, the chemical state of groundwater is assessed as “poor’, using maximum admissible concentrations as threshold values for potable use, due to high concentrations of nitrate and ammonium ions.
These findings are in accordance to the human activities taking place in the wider area. The intensive cultivation practices exercised for many decades include excessive application of nitrogen fertilization and are the main reason that has led to the documented nitrates levels in the examined aquifer system. Implementation of appropriate Action Plans against nitrates pollution of agricultural origin and adoption of compiled Codes for Good Agricultural Practices are thought to progressively improve the current conditions. Towards this direction, systematic monitoring of both soils and water resources ought to be implemented as a means of evaluating the performance of the alleviation measures taken. It has to be stressed that the documented chemical state of the examined groundwater body also reflects its susceptibility to pollution, the level of which is also affected by the poor hydrological conditions experienced in the region during the study period. Lower natural recharge to the system and increased groundwater abstractions for irrigation purposes have resulted in progressive concentration of the pollutants is lower groundwater volumes. The fact that in general the chemical quality of the underlain karstic aquifer system does not exhibit signs of systematic pollution originating from agricultural activities, stresses the importance of the sedimentary aquifer system as a buffer zone.
There is an immediate need for integrated water resources management in the region with emphasis on restoring the chemical quality of affected water bodies. This is dictated not only by the relevant EU Directives and policies on the protection and management of water resources, but mainly by the need to ensure sufficient and safe access for all end-users including the environment. Furthermore, under the new Common Agricultural Policy of the EC, clean environment is an essential prerequisite along with clean production in shaping up a strong market of high added value agricultural products. Hence, in an agricultural region as the study area is, the road to socioeconomic stability inevitably goes through environmental protection.
Acknowledgement
The data of this study resulted from chemical analyses carried out within the research project LIFE07 ENV/GR/0000266 EcoPest, entitled “Strategic Plan for the adaptation and application of the principles for the sustainable use of pesticides in vulnerable ecosystem.” The authors wish to acknowledge the valuable contribution of the scientific team of the Soil and Water Resources Institute (former Land Reclamation Institute), who worked on this project for the production of the presented hydrochemical data.
References
- Karyotis Th, Panagopoulos A, Arabatzis G, Charoulis A, Domakinis C, Tziouvalekas M, et al. Seasonal variation and chemical characteristics of groundwater and quality assessment in the intensively cultivated Voiotia Plain, Greece. E-Proc. 11th ICOBTE International Conference on the Biogeochemistry of Trace Elements. Florence, Italy. 2011; July 3- July 7.
- Domakinis C, Panagopoulos A, Arampatzis G, Charoulis A and Karyotis T. Developing drastic for an intensively cultivated vulnerable basin of Greece. E-Proc. X International Conference on Protection and Restoration of the Environment. Corfu, Greece. 2010; July 5- July 9.
- Dandolos I, Zorapas V. Definition and assessment of groundwater aquifer systems of Kopais Basin and sub basins of Viotia – Evia prefectures. Final Report: Water District of East Central Greece (07), IGME, Athens. 2010.
- Konstantinou V. Groundwater quality in a part of Viotikos Kifisos River Basin. Postgraduate Thesis, School of Biology, Aristotle University. Thessaloniki. 2013.
- Mountrakis D. Geology of Greece, University Studio Press, Thessaloniki. 1985.
- Panagopoulos A, Arampatzis G, Vrouhakis I, Panoras A. Leachate monitoring network design in the vadose zone at Viotikos Kifisos basin, central Greece. Proceedings of the?6th International Congress?of European Society for Soil Conservation, Innovative Strategies and Policies for Soil Conservation. 2011; May 9- May 14, Thessaloniki. 2011; 138-139.
- Panagopoulos A, Kassapi K, Arampatzis G, Perleros B, Drakopoulou S, Tziritis E, et al. Assessment of chemical and quantitative state of groundwater systems in Pinios hydrological basin, Greece. International Conference on Protection and Restoration of the Environment XI. 2012; July 3- July 6, Thessaloniki. 2012; 511-517.
- Official Gazette of the Greek Government. Modification of JMD ?2/2600/200 “Quality of water intended for human consumption” in compliance with Directive 98/83/EU of the Council of the European Union on November 3, 1998. Legislation. JMD2/G.P. Fin. 38295, Sheet No. 630, Athens. 2007.
- Official Gazette of the Greek Government. Set threshold values for concentrations of specific pollutants, groups of pollutants or indicators of pollution in groundwater. Legislation, JMD, No. Fin.1811, Sheet No. 3322, Athens. 2011.
- Directive 2000/60/EC of the European Parliament and of the Council establishing a framework for Community action in the field of water policy. Legislation, Official Journal of the European Union, L. 2000; 327: 1-72.
- Directive 2006/118/EC of the European Parliament and of the Council on the protection of groundwater against pollution and deterioration, Legislation, Official Journal of the European Union, L. 2006; 372:19-31.
- Tziritis E. Groundwater and soil geochemistry of Eastern Kopaida region, (Beotia, central Greece). Central European Journal of Geosciences. 2009; 1: 219-226.
- Tziritis E. Assessment of NO3- contamination in a karstic aquifer, with the use of geochemical data and spatial analysis. Environmental Earth Sciences. 2010; 60: 1381-1390.
- Voudouris K, Panagopoulos A, Koumantakis I, Nitrate pollution in the coastal aquifer system of the Korinthos prefecture (Greece). Global Nest: the International Journal, Greece. 2004; 6: 31-38.
- Matschullat J, Ottenstein R, Reimann C. Geochemical background–can we calculate it. 2000; 39: 990-1000.
- Reinman C, Garret R G. Geochemical background – concept and reality. Science of the Total Environment. 2005; 350: 12-27.
- Christoforidou P. Derivation of Natural Background levels and threshold values definition in groundwater bodies of Greece. Thesis. School of Biology, Aristotle University, Thessaloniki, 2009.