
Research Article
Austin J Hydrol. 2016; 3(1): 1019.
Hydrochemistry and Environmental Isotopes to Identify the Origin of Barapukuria Coal Mine Inflow Water, Northwestern Bangladesh
Majumder RK1* and Shimada J2
1Nuclear Minerals Unit, Bangladesh Atomic Energy Commission, Bangladesh
2Graduate School of Science and Technology, Kumamoto University, Japan
*Corresponding author: Majumder RK, Nuclear Minerals Unit, Bangladesh Atomic Energy Commission, Savar, Dhaka 1349, Bangladesh
Received: April 10, 2016; Accepted: May 23, 2016; Published: May 25, 2016
Abstract
Groundwater, coal mine inflow water, and river water samples were collected during November and December 2006 from the Barapukuria coal mine area in Dinajpur District, northwest Bangladesh. Groundwater samples were collected from existing shallow wells. All water samples were analysed for major ions, stable oxygen and hydrogen isotopes, and for tritium. High Electrical Conductance (EC) values, alkaline pH, and high temperature of collected coal mine inflow water signify a good hydraulic connectivity between the most fractured coal seam bearing Gondwana aquifers and the overlying Dupi Tila aquifers. Both groundwater and coal mine inflow water were dominantly of Na–Ca–HCO3 and Ca–Na–HCO3 type. The clustering of groundwater and coal mine inflow water samples along the Local Meteoric Water Line (LMWL) within narrow range indicates a common origin for the collected water samples, and thus indicates that the groundwater and coal mine inflow water originate from local rainfalls. The similarity between the average δ18O composition of groundwater and coal mine inflow water indicates that the groundwater percolates into the coal seam bearing Gondwana aquifers without changing recharging groundwater δ18O compositions. Both the groundwater and mine water tritium values were <1.0 TU, which could be considered as old water recharged prior to 1952. Finally, it can be concluded that the Barapukuria coal mine inflow water is of meteoric origin, which have been recharged within the aquifers prior to 1952 and there is connectivity between the shallow Dupi Tila aquifer and the coal seam bearing Gondwana aquifer.
Keywords: Groundwater; Coal mine water; Hydrochemistry; Environmental isotopes; Barapukuria
Introduction
Barapukuria is the first and only coal mine in Bangladesh and is sited on a subcropped asymmetrical synclinal deposit of Permian age Gondwana coal measures. The structure was first indicated by a negative gravity anomaly in oil and gas exploration initiated by the Geological Survey of Bangladesh (GSB) with seven surface boreholes. Within the structural limits of the coal basin, approximately 377 Mt coal in-situ has been quantified in the six coal seams that range in depth from 118 to 518 m below surface (Figure 1). Due to the synclinal nature of the deposit, the upper coal seams, designated I to V, occur over diminishing areal extent with decreasing depth. The principal seam of interest is the lowermost Seam VI, with a variable thickness across the deposit from 22 m in the northern part of the deposit to more than 42 m in the southern and eastern areas [1]. In 1994, the Bangladesh government signed a contract with the Chinese contractor CMC (China National Machinery Import and Export Corporation) for the development of Barapukuria coal mine by underground mining method [2]. The development of the Barapukuria coal mine commenced in 1996 with the construction of two vertical shafts. Coal production from Seam VI began in 2005 and has been continued at the present time. About 34 Mt of coal has been estimated as recoverable resources, utilising descensional multi-slice long wall mining [1].
\

Figure 1: Structure, stratigraphy, and distribution of coal seams of the Barapukuria coal basin [22]. Seams II, IV, V, and VI are clearly visible in Figures a, and. c.
Seams I and III are not shown in these sections due to small-scale and variable thickness.
The mine design and development have been severely constrained by adverse seam gradients and the presence of the overlying waterbearing Tertiary Dupi Tila sediments. As for the exploration report, the hydrogeological condition around the mine is much complicated as a result the mine industry endured various problems to develop new roadway and safely take out coal from underground. During the development work of coal mine in 1996, a severe water inrush accident occurred consequently thoroughly inundated the underground roadway. In this case, this waterlogged condition has been resolved using forced pumping activities [3] and the Barapukuria coal mine operated by the Barapukuria Coal Mining Co. Ltd. (BCMCL) requires continuous pumping and discharge of nearly 1,500 m3 of water every hour to keep the mine free from flooding [4,5].
Mining activities below water table trigger the inflow of groundwater to the pit and creates many difficulties and hazards most likely increased drilling and blasting costs, difficulties in ore handling and crushing, decreased machinery life, slope instability, degradation of water quality, and environmental problems [6]. Therefore, management of groundwater and planning of appropriate dewatering systems are imperative requirements for safe, sustainable, and cost effective mining below the water table. Adequate management of groundwater [7,8] and controlling groundwater inflows to the mine cuttings [9] requires good understanding of the sources of recharge and the major groundwater flow paths. It is important to understand the source, flow paths and residence time of groundwater in and around the Barapukuria coal mine for mine water management as well as for future mine development. Environmental isotope and hydrochemical studies can provide useful complementary information in aquifers with complex lithology [10-12].
Environmental stable isotopes (18O, 2H) are commonly used in groundwater studies to identify flow regimes and sources of recharge [13-15]. Frequently, groundwater retains its stable isotopic signatures unless diluted or mixed with waters of different isotopic composition [16,17]. Therefore, waters from different sources or those exposed to different processes such as evaporation and/or mixing, often acquire identifiable isotopic contents, which can serve as natural tracers [18]. Radiogenic 3H isotope can be used to evaluate different residence time of the waters and mixing processes. Tritium (3H) is a naturally occurring radioactive isotope of hydrogen and part of water molecule; its concentration is not affected significantly by reactions other than radioactive decay. Detectable 3H concentrations in groundwater give the evidence that recharge has occurred after nuclear bomb tests (1952–1953) or mixing had occurred between recent tritiated water and water recharged prior to 1950s [19]. In present study, isotope values as well as hydrochemical concentrations of rainwater, river water, groundwater and mine water have been studied systematically i) to ascertain the hydrochemical evolution of groundwater in and around the coal mine aquifers, ii) to determine the origin and time scales of recharge, and groundwater and mine water residence time, and iii) finally, the research results would help to manage and control the groundwater inflows to the mine cuttings which would be required for the development of proper dewatering systems of the Barapukuria coal mine.
Study Area
Geology and hydrogeology
Barapukuria coal basin, covering an area of approximately 5.16 km2 within a wide flood plain, is located in Dinajpur District of northwestern part of Bangladesh (Figure 2). The mine area lies between latitudes 25º31′45′′N and 25º33′05′′N, and longitudes 88º57′48′′E and 88º58′53′′E (Figure 2). The coal mine area and its surroundings lie in the northern part of the Barind tract and are characterized by a great table land of Pleistocene terraces with low relief. The average elevation of ground level is 30.5 m above the Average Mean Sea Level (AMSL) and has a regional slope from north to south with a gradient of 0.45 meters per kilometre [20]. The Barind tract is extensively dissected, with narrow or broad valleys extending deep into the level landscape. The valleys are cultivated with rice in the dry season by impounding the streams for irrigation [21].
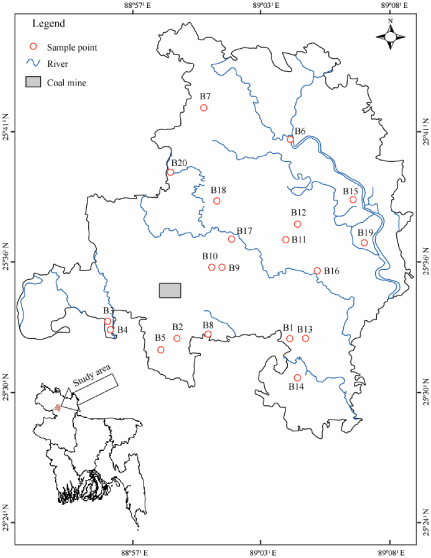
Figure 2: Study area map showing well sites and coal mine area.
The geologic structure and stratigraphy of the Barapukuria coal basin have been delineated with the help of borehole data and seismic survey by the Geological Survey of Bangladesh and Wardell [22]. The study area consists of a half faulted graben controlled sedimentary basin of Permo-Carboniferous age. The lower most Permo-Carboniferous glacial and fluvioglacial sediments (i.e., tillite) resting unconformable on a denuded Archaean basement complex. Above this tillite, thick predominantly continental arenaceous sediments with a number of coal seams of Permian age are present. This basin is concealed by an unconformable cover of about 100-200 m of Tertiary sediments known as the Dupi Tila Formation, which is supposed to be of Pliocene age [23,24]. The Barapukuria basin area is a plain land covered with Recent Alluvium and Pleistocene Barind Clay Residuum. The stratigraphic succession of this basin has been established on the basis of borehole data [23]. The sedimentary rocks of the Permian Gondwana Group, Pliocene Dupi Tila Formation, Pleistocene Barind Clay Residuum and Recent Alluvium were respectively encountered in the boreholes, which lie on the Archaean Basement Complex. A large gap in sedimentary record is present in between Gondwana Group and Dupi Tila Formation, which is probably caused by the erosional or non-depositional phase, existed during Triassic to Pliocene age. The stratigraphic succession in the Barapukuria coal basin is given in Table 1.
Age
Group
Formation
Member
Lithology
Max. thickness (m)
Holocene
Alluvium
Silty clay
1.83
Pleistocene
Barind Clay Residuum
10.36
Pliocene
Dupi Tila
Upper
Sandstone, pebbly sandstone and clay/mudstone
126.82
Lower
Sandstone, claystone and mudstone with silica and white clay
Permian
Gondwana
Feldspathic sandstone, carbonaceous sandstone and shale, ferruginous sandstone, conglomertes, and coal beds.
457.32
Precambrian
Basement Complex
Diorite, granodiorite, quartzdiorite, granite, and diorite gneiss.
14.32+
Table 1: Stratigraphic succession of the Barapukuria Basin.
Barapukuria coal mine is called mine under water, because of the presence of thick water-bearing formation over the recoverable coal seam where groundwater flows from NE to SW direction having almost flat (0.0004 to 0.0006) hydraulic gradient and horizontal flow is insignificant compared to vertical flow [22]. The groundwater sources of the study area are mainly the Upper Dupi Tila Formation and the Gondwana Formations.
In the study area, Barind clay acts as an aquiclude and mainly consists of brownish yellow sandy clay, interbedded with dark gray silt to fine sand in lower part, occasionally appears bluish gray argillaceous clay, and composed of gray clayey silt at the base. This aquiclude is thick in the north-west and thin in the south-east, with a thickness ranging from 4.3-10.36 m. The Upper Dupi Tila (UDT) aquifer underlying the Barind Clay has regional extension with high water bearing capacity and acts as the main water recharge source for various aquifers in Gondwana strata and for coal mine inflows. The UDT aquifer is thin in the south and north while thick and deep in the centre eastwards, with an average thickness of 104.41 m and depth to the floor of this aquifer varies from 102.7–136.2 m below the surface. This hydrologic unit is mainly composed of medium sand beds interbedded with fine sand, pebbly grit and thin clay horizons where clay content increases with the depth.
The Lower Dupi Tila (LDT) is an aquiclude and it consists of gray-white weathered clay and clay siltstone. Thickness of which varies from 0 to 80.14 m and the bottom surface is consistent to the relief of Gondwana strata. The elevation of the upper surface of LDT ranges from 80 to 90 m below the ground surface and deepens progressively in the south-western and south-eastern parts up to depth of 80 to 160 m below the ground surface. About 78% of coalbearing strata underlies unconformably by the LDT. The Gondwana Sandstones represent completely an aquifer system and the coal seam VI divides this as upper and lower sections. The Upper section is a sandstone aquifer with a thickness of 0 to 156 m, only remained in the centre of the basin. The lithology mainly consists of medium to coarse grained sandstones and pebbly sandstone, interbedded with coal seams, siltstone and mudstone. In the Upper Gondwana section, vertical tensile cracks mostly filled with mud or pyrite films are well developed [25]. The Lower Gondwana section located at floor of coal seam VI and the thickness of this aquifer varies from 107 to 244 m, and becomes thick in the centre of the basin from west-north to eastsouth. It consists of medium to coarse grained sandstone, gritstones and conglomerates, interbedded with mudstone and siltstone.
Rainfall, recharge and drainage system
The analyses of ten years (2000–2009) rainfall records in Barapukuria Coal Mine area revealed that the maximum and minimum annual rainfall were 2955 mm (2006) and 1166 mm (2008), respectively. Throughout the years 2000–2009, about 88% of the annual rainfalls occurred in monsoon and pre monsoon (March– October) and the remaining 12% in the winter season (November- February). According to analysis of the dynamic relationship curve between underground water level and rainfall [25], rainfall is the main source for water recharging in the study area aquifers. The study area is drained in the western side by the Khorkhori River, which flow almost north-south direction. Another river the little Jamuna (local name) flows in the western side of the river Khorkhori. The river Ghirnai flows through the north-eastern side of the study area, which remains almost dry during the winter season and becomes navigable in the rainy season.
Methodology
Field sampling
In present study, nineteen groundwater (depth ranging from 11–61m), eight coal mine water and one river water sample were collected during November-December 2006 (Figure 2). Prior to sampling each well was pumped for several minutes until steady state physical conditions (pH, electrical conductivity and temperature) were obtained. The geographical location of each well was determined with a GARMIN handheld Global Positioning System (GPS) and the depth of wells were noted from the well owner’s records. The physical parameters such as electrical conductivity, pH and temperature were measured with a portable EC/pH meter (TOA EC/pH METER, WM– 22EP). The samples for major ion analysis were filtered by 0.25μm polycarbonate filters. For 3H analysis one litre of groundwater sample was collected in High Density Polyethylene (HDPE) bottles. Unfiltered aliquots were collected for δ18O and δ2H analysis in 100 ml HDPE bottle [12,18,26,27]. Prior to shipment to Japan by express mail, all the samples were preserved in room temperature in Isotope Hydrology Laboratory, Institute of Nuclear Science and Technology, Bangladesh Atomic Energy Commission, Dhaka, Bangladesh and later on the samples were shipped to Japan and stored in room temperature in Hydrology Laboratory, Kumamoto University, Japan until the analysis being performed.
Laboratory analyses
The concentrations of anions (Cl-, NO3 - and SO4 2-) and cations (Na+, K+, Ca2+ and Mg2+) in water samples were measured using ion chromatography (Compact IC, 761, Metrohm). The instrument was linearly calibrated with standards (Wako Pure Chemicals Industries Ltd., Japan) in Kumamoto University, Japan [27]. Alkalinity (as HCO3 -) was measured by titration method in the same laboratory [12].
Oxygen isotope ratios (18O/16O) of water samples were determined in Hydrology Laboratory, Kumamoto University, Japan using CO2 equilibration method and the chromium reduction method was used for the determination of 2H/1H ratios, followed by analysis with a Thermo Electron Delta S mass spectrometer using conventional techniques [28]. Hydrogen and oxygen isotope ratios are expressed by δ2H and δ 18O, respectively, where δ = [(Rsample/Rstandard) –1] 1000 (‰), R is the ratio of 2H/1H or 18O/16O in sampled water (Rsample) or in Standard mean ocean water (Rstandard). The isotopic compositions were reported in standard δ–notation representing per mil (‰) deviations from the V–SMOW standard (Vienna Standard Mean Ocean Water) [29]. The analytical errors were ±0.1‰ for δ18O and ±1.0 ‰ for δ2H. Tritium determination was performed with a liquid scintillation counter (TRI–CARB2750TR/LL, Perkin–Elmer Co.) after electrolytic enrichment [30]. The results were reported as Tritium Units (TU), i.e., one atom of tritium in 1018 atoms hydrogen. The values of physical parameters, major ion concentrations, and 3H, δ18O and δ2H compositions of collected water samples are shown in Table 2.
Sample ID
Sample Type
Depth
(m)
pH
Temp.
(°C)
EC (mS/cm)
Cl (mg/L)
SO4 (mg/L)
NO3
(mg/L)
HCO3 (mg/L)
Na (mg/L)
K (mg/L)
Mg (mg/L)
Ca
(mg/L)
d18O
(‰)
d2H
(‰)
3H (TU)
Water
Type
B1
Groundwater
12
6.29
26.4
340
10.57
25.14
41.64
120
23.01
9.81
8.16
22.49
-5.4
-34
4.5
Ca-Na-HCO3
B2
-do-
11
6.58
26.1
272
14.89
3.57
0.63
193
10.11
4.85
10.73
30.06
-3.4
-21
?
Ca-Mg-HCO3
B3
-do-
61
6.90
27.2
140
1.34
0.52
0.51
133
16.01
1.72
3.29
7.78
-5.3
-34
0.6
Na-Ca-HCO3
B4
-do-
18
6.83
26.7
86
1.15
0.36
0.28
78
6.50
1.60
2.65
5.21
-5.3
-33
?
Na-Ca-HCO3
B5
-do-
23
6.20
26.3
233
47.50
0.34
0.55
105
19.08
1.81
6.28
16.70
-4.8
-31
?
Ca-Na-HCO3
B6
-do-
18
6.91
26.3
159
1.15
0.84
0.55
146
18.72
2.00
3.20
9.75
-4.8
-30
0.4
Na-Ca-HCO3
B7
-do-
27
6.83
26.7
168
12.20
0.34
0.51
146
16.63
2.58
3.89
11.11
-5.2
-32
?
Na-Ca-HCO3
B8
-do-
20
6.74
27
238
21.60
0.77
0.84
133
17.59
0.92
5.49
17.47
-4.7
-30
?
Na-Ca-HCO3
B9
-do-
23
6.70
26.4
137
7.09
0.73
0.75
109
13.25
0.47
2.96
7.27
-5.1
-34
0.5
Na-Ca-HCO3
B10
-do-
61
6.81
26.7
221
6.36
2.52
0.83
230
16.20
0.10
7.72
14.92
-4.8
-31
?
Ca-Na-HCO3
B11
-do-
61
6.89
26.1
144
4.38
1.01
0.76
121
8.54
2.23
3.90
11.58
-5.2
-35
?
Ca-Na-HCO3
B12
-do-
24
6.85
26
298
29.05
4.90
0.86
172
15.23
2.82
8.07
26.78
-4.0
-26
0.3
Ca-Mg-HCO3
Table 2: Physical parameters, major ions and environmental isotopic compositions of collected water samples.
B13
-do-
23
6.82
26.5
156
2.15
0.08
0.70
137
14.28
1.06
3.29
11.05
-4.2
-31
?
Na-Ca-HCO3
B14
-do-
34
6.91
26.3
205
2.89
0.04
0.76
203
17.35
1.47
6.98
14.29
-4.0
-28
?
Na-Ca-HCO3
B15
-do-
30
7.00
27
620
1.13
0.06
0.65
47
8.08
0.98
1.56
2.40
-5.2
-36
?
Na-Ca-HCO3
B16
-do-
29
6.85
28.2
176
1.28
0.10
0.80
156
14.45
1.21
5.10
13.92
-4.8
-32
?
Ca-Na-HCO3
B17
-do-
23
6.90
26.2
167
4.97
0.27
0.76
262
13.31
2.90
4.57
13.97
-4.1
-28
?
Ca-Mg-HCO3
B18
-do-
23
6.80
26.2
168
1.03
0.39
0.75
172
17.09
1.38
4.20
10.78
-4.6
-31
?
Na-Ca-HCO3
B19
-do-
20
6.64
26.8
202
28.16
10.53
29.01
137
17.21
4.35
5.23
23.52
-5.2
-34
?
Ca-Na-HCO3
B20
River water
0
7.62
30.3
510
1.61
1.01
0.32
35
2.51
3.09
1.15
3.20
-9.3
-66
?
Ca-Na-HCO3
B21
Mine water
260
8.10
37.1
1250
9.86
24.01
0.66
62
25.28
1.67
5.18
22.36
-4.8
-28
0.6
Ca-Na-HCO3
B22
-do-
260
8.26
37.2
1251
8.64
23.34
0.49
55
24.47
3.06
4.30
14.35
-4.8
-28
?
Na-Ca-HCO3
B23
-do-
260
8.22
37.1
1250
4.74
26.87
0.80
62
24.33
2.77
2.04
15.22
-4.7
-29
?
Na-Ca-HCO3
B24
-do-
260
8.25
37.0
1253
5.45
27.70
0.75
86
25.15
3.03
1.03
20.12
-4.6
-28
0.5
Na-Ca-HCO3
B25
-do-
260
8.00
37.1
1251
5.44
27.16
0.86
82
23.98
4.88
5.04
18.87
-4.6
-28
?
Na-Ca-HCO3
B26
-do-
260
8.00
37.0
1252
4.87
23.89
0.69
49
25.62
4.67
0.80
13.89
-4.4
-27
0.7
Na-Ca-HCO3
Table 2: (2of1) Physical parameters, major ions and environmental isotopic compositions of collected water samples.
Results
Hydrochemistry
Electrical Conductance (EC) is an indication of the dissolved mineral content of groundwater. Groundwater Electrical Conductance (EC) appeared to increase from shallower groundwater sample towards the deeper coal mine. The EC values of the observed groundwater samples ranged between 86 μS/cm and 620 μS/cm with an average value of 230 μS/cm and the coal mine water average EC value was 1268 μS/m ranging from 1233 μS/m to 1553 μS/m. The average EC value of the coal mine water samples was much higher (more than 5 times) than the groundwater average EC value. Groundwater pH is a fundamental property that describes the acidity and alkalinity of groundwater and largely controls the amount of chemical form of many organic and inorganic substances dissolved in groundwater. Groundwater pH is related to CO2 and HCO3-. Carbon dioxide dissolves slightly in water to form carbonic acid (H2CO3), according to the following reaction: CO2 + H2O→ H2CO3 and thus gives raise to acidic pH values (<7). The pH of groundwater increases as carbonic acid (i.e., CO2) is removed from groundwater according to reactions: H2CO3→ H+ + HCO3 - followed by HCO3 - + H+→ H2O + CO2. The groundwater pH values ranged from 6.2–7.0 with an average value of 6.75. However, coal mine water average pH value was alkaline (8.15) ranging from 8.0–8.26. Groundwater temperature is considered to be one of the most easily measurable, natural tracers of groundwater flow [31]. The observed groundwater average temperature was 26.6 °C ranging from 26–28.2°C, while the coal mine water temperature varied from 37–38°C with an average value of 37.3 °C.
In collected groundwater samples, the average concentrations of Na+, K+, Ca2+ and Mg2+ were 14.86 mg/L (ranged from 6.50–23.01 mg/L), 2.58 mg/L (ranged from 0.10–9.81 mg/L), 14.45 mg/L (ranged from 2.40–30.06 mg/L) and 5.22 mg/L (ranged from 1.56–10.73 mg/L), respectively. Meanwhile, the average Cl–, SO4 2–, HCO3 – and NO3 –concentrations of groundwater sample were 12.78 mg/L (ranged from 1.03–12.78 mg/L), 3.70 mg/L (ranged from 0.04–25.14 mg/L), 5.91 mg/L (ranged from 0.28–41.64 mg/L) and 148 mg/L (ranged from 47–262 mg/L), respectively. The mine inflow water Na+, K+, Ca2+ and Mg2+ concentrations ranged from 21.84–65.61 (average 36.21 mg/L), 1.67–11.56 (average 5.11 mg/L), 13.89–41.37 (average 22.37 mg/L) and 0.79–11.91 mg/L (average 4.97 mg/L), respectively. In addition, mine water average Cl–, SO4 2–, HCO3– and NO3 – concentrations were 5.47 mg/L (ranged from 1.93–9.86 mg/L), 21.56 mg/L (ranged from 1.56–29.79 mg/L), 0.73 mg/L (ranged from 0.49– 0.92 mg/L) and 70 mg/L (ranged from 49–88 mg/L), respectively. The trend of major cation concentrations in groundwater and mine water were Na+>Ca2+>Mg2+>K+ and Na+>Ca2+>K+>M2+, respectively. The groundwater was of HCO3 - dominant followed by NO3-, SO4 2- and Cl-, whereas the mine water was also HCO3 - dominant followed by SO4 2-, Cl- and NO3-.
Piper plot
Piper diagrams [32] are widely used to present and classify major ions for groundwater types and summarize the main contrasts in hydrochemical composition between different water sources [33]. The hydrochemical water types of groundwater, coal mine water and river water are conducted by a Piper triangular diagram (Figure 3) and water types are shown in Table 2. Ca–Na–HCO3 type was observed in river water (B20) and six groundwater samples Table 2. But low mineralized Ca–Mg–HCO3 type water was found only in three groundwater samples (B2, B12 and B17), whereas slightly mineralized Na–Ca–HCO3 type water was found in rest of the groundwater samples. For coal mine water, Na–Ca–HCO3 type water was observed in five samples (B22, B23, B24, B25 and B26), Ca–Na– HCO3 type in two samples (B21 and B27) and low mineralized Ca– Mg–HCO3 type water only in one sample (B28).
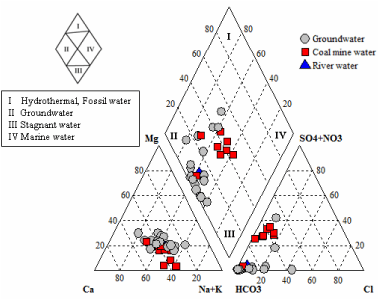
Figure 3: Major ion composition of groundwater, coal mine water and river
water samples plotted on a Piper diagram.
Oxygen, hydrogen and tritium isotopes
The collected groundwater sample d18O and d2 compositions ranged from –5.4 to –3.4‰ (average –4.7‰) and –36 to –21‰ (average –31‰), respectively. Meanwhile, the d18O and d2 compositions of collected coal mine water samples ranged from –5.0‰ to –4.4‰ (average –4.7‰) and –32 to –27‰ (average –28.9‰), respectively. Surprisingly, the average d18O (–4.7‰) composition of coal mine water was similar to the groundwater average d18O value (–4.7‰). But the river water d18O (–9.3‰) and d2 (–66‰) compositions were much lighter than the average groundwater and mine water d18O and d2 compositions Tritium (3H) is a natural radioactive isotope of hydrogen which is formed naturally by the dissociation of nitrogen following interaction with cosmic rays. It is also formed as a byproduct of thermonuclear testing and has a half life of 12.26 years. The widespread testing ‘above ground’ of thermonuclear devices between 1952 and 1962 drastically increased the amount of tritium in the atmosphere swamping natural background levels. The intermittent testing of such devices has also led to the introduction of periodic pulses or peaks of tritium to the atmosphere. Over land areas the removal of atmospheric tritium is principally via precipitation, which is extremely variable and dependent on the various climatic, land distance and orographic influences affecting precipitation. It is therefore apparent that tritium concentrations reaching the earth’s surface can be exceedingly variable in both time and space. Consequently, tritium levels in groundwater via both direct and indirect recharge can also be extremely variable [34]. Tritium content of analysed groundwater and coal mine water ranged from 0.1 to 4.5 TU.
Discussion
The composition of mineral salts affects the Electrical Conductivity (EC) of groundwater and EC values of groundwater increase along flow paths with increasing groundwater residence time, whether groundwater is not affected by sea water intrusion [18]. The high EC values (more than 5 times) for the coal mine water collected from the dewatering chambers indicate a good hydraulic connectivity between the most severely fractured coal seam bearing Gondwana sandstone and the overlying Dupi Tila aquifers. It also signifies that the fractured Gondwana sandstone permeability greatly increases downward and drains directly into the coal mine. Thus the long migration path of the infiltrating groundwater facilitates dissolution of mineral salts along the fractured Gondwana sandstone which may cause higher EC values and these results comply with the conclusion made by Kibria et al. [2]. The acidic to neutral groundwater pH values imply an open system unconfined aquifer all over the study area with high soil CO2 giving rise to low pH. The alkaline pH values (>8.0) for coal mine water suggest decline in partial pressure of CO2 (pCO2) levels within the Gondwana aquifers and indicates lack of oxygen in the deeper Gondwana aquifers which could contribute to a more alkaline pH [35] for the inflowing coal mine water.
Circulating groundwater will acquire geothermal heating along its flow path [36] and subsurface temperature acts as a tracer for detecting the groundwater movement [37]. In present study the coal mine water average temperature (37.3 °C) is much higher (more than 10.7 °C) than the groundwater average temperature (26.7 °C). This implies that groundwater having low temperature within the Dupi Tila aquifers percolates into the deeper Gondwana aquifers attaining high temperatures within the coal seams which cause high heat flow in the coal mine [38].
Piper diagram shows that most of the samples are clustered at the central diamond (group-II) (Figure 3). The Piper diagram illustrates that the groundwater is dominantly of Na–Ca–HCO3 and Ca–Na– HCO3 type water, which are the initial source of water recharging into the aquifer systems. The Na–Ca–HCO3 type groundwater observed in the study area shows slightly increase of Na+ concentration with respect to Ca2+ and Mg2+. The increase in Na+ exchange for Ca2+ and Mg2+ suggest softening process, which may indicate rapid recharge and/or much more water-rock interactions along the flow paths. The coal mine water samples are also rich in Na–Ca–HCO3 and Ca–Na–HCO3 type water and this phenomenon indicates possible connectivity between overlying Dupi Tila and underlying Gondwana aquifers causing rapid recharge into the Gondwana aquifers without changing chemical characteristics of recharging water. Besides, Ca– Mg–HCO3 type low mineralized water observed in both groundwater and coal mine water also indicates connectivity between the shallow and deeper coal seam bearing Gondwana aquifers.
The deeper the groundwater moves, the more enriched it becomes in Na as more dissolution and cation-exchange takes place [39]. In present study, the Na concentration of groundwater samples ranged from 6.50–23.01 mg/L with an average of 14.86 mg/L. The low Na concentration in collected groundwater suggest a relatively short residence time (greater transmissivity) from the Dupi Tila aquifer. The coal mine water Na concentration ranged from 21.88– 65.62 mg/L with an average of 36.21 mg/L. The relatively high Na concentration of coal mine water indicates that this water is derived from groundwater moving slowly through the shallow Dupi Tila aquifer and reacts with Na-rich minerals through dissolution. Finally, a preferred conduit and enhance comminution of rock fragments along the fractured Gondwana rocks promote Na dissolution giving rise to high Na concentrations in coal mine water.
The average Ca2+ and Mg2+ concentrations of groundwater samples were 14.45 mg/L and 5.22 mg/L, respectively. Meanwhile, coal mine water average Ca2+ and Mg2+ concentrations were 22.38 mg/L and 4.98 mg/L, respectively. It is obvious that coal mine water average Ca concentration is nearly 1.6 times higher than that of groundwater average Ca2+ concentration. But the average Mg2+ concentration of coal mine water is to some extent lower than that of groundwater average Mg concentration. It indicates that surface water draining towards and into the coal mine does not undergo sufficient base exchange reactions and thus Ca2+ and Mg2+ are not enough adsorbed onto clays within the sediments in exchange for Na+ [40]. Thus the interstitial release of Na from the overlying Gondwana sediments occurs as water flows down through the fractured Gondwana sediments with insignificant cation exchange as a function of relatively short distance that the water has travelled [41] as well as short residence time. It is evident that the resultant water that ends up on the coal seam horizon is slightly enriched in Ca2+ and less enriched in Mg2+, and it indicates calcite dissolution along the flow path rather than dolomite dissolution.
In groundwater, the cations Ca2+ and Mg2+ often come from carbonate minerals such as calcite and dolomite. Most of the major cations released in groundwater from carbonate minerals dissolution enhanced by respired CO2 from oxic and anoxic organic matter degradation [42]. Reactions that specifically produce CO2 through oxidation of organic matter are generalized by:
CH2O (organicmatter) + O2→ H2O + CO2 (1)
This reaction is then followed by
CaMg(CO3)2 + CO2 + H2O → Mg2+ + 2HCO3 - +CaCO3 (2)
CaCO3 +CO2 + H2O → Ca2+ + 2HCO3 - (3)
resulting in the high Ca2+, Mg2+ and HCO3 - concentrations found in groundwater. There is also CO2 in rain water which can facilitate carbonate mineral dissolution [43]. So that there would be straight positive correlation between Ca2+ and HCO3 –, and Mg2+ and HCO3 –. A bi-variant plot (Figure 4) of Ca2+ versus HCO3 – shows strong positive correlation (r2=0.60) for groundwater, whereas Mg2+ versus HCO3 – shows weak correlation (r2=0.15) for mine water (Figure 5). This indicates that groundwater in Dupi Tila aquifer moves slowly downward through the Gondwana aquifer with minor dissolution of calcite and latter have a low Ca+ concentration due to relatively short residence times within the aquifer systems.
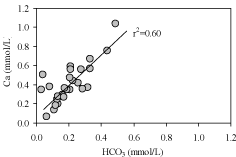
Figure 4: Bivariate plot of Ca versus HCO3.
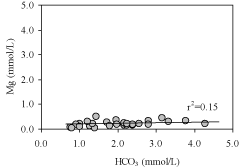
Figure 5: Bivariate plot of Mg versus HCO3.
The average SO4 2- concentration of coal mine water is nearly six times higher than the groundwater SO4 2- concentration. The elevated concentration of SO4 2- in mine water can be attributed to the oxidation of sulphate minerals within the coal mine [44]. Meanwhile, the elevated sulphate concentrations in coal mine water samples indicate that the coal seam has a high acid-generating potential [41]. Nevertheless, the alkaline pH level of the coal mine water suggests that the coal seam also has sufficient base potential to neutralise the generated acid within the mine.
In Bengal delta aquifers, the sources of HCO3 – would probably include root respiration (soil zone CO2), microbial degradation of organic matter and carbonate dissolution [45]. In present study, the groundwater average bicarbonate concentration is more than two times higher than that of the coal mine water bicarbonate concentration having low pH (average <6.75). The relatively high concentrations (average 148 mg/L) of HCO3 – in the shallow unconfined Dupi Tila aquifer groundwater may be caused by CO2 dissolution of local meteoric water [46] and carbonate dissolution rather than microbial degradation of organic matter. The soil zone in the study area may contain elevated CO2 pressure produced by decay of organic matter and root respiration, which in turn combines with rainwater to form bicarbonate following the reactions CO2 + H2O → H2CO3 and H2CO3 → H+ + HCO3 – [47]. Bicarbonate may also be derived from the dissolution of calcite (Figure 4) rather than dolomite (Figure 5) following the equation (i). According to Kinniburgh and Smedley [48], Dupi Tila aquifers were thoroughly oxidized during its geological evolution causing a decrease in organic matter concentration in the aquifers. In addition, Ueno [49] found that bacteria may be consumed organic matters more easily from younger Holocene sediments than that of older Pleistocene Dupi Tila sediments. As per the above mentioned statements, it is obvious that the presence of insignificant concentrations of organic matter in Dupi Tila aquifer sediments may not be responsible for releasing higher concentration of HCO3 – in groundwater released by the microbial degradation of organic matter. The low HCO3 – concentrations of coal mine water compared to groundwater, are attributed to a lack of CO2 present (confined aquifer), resulting slow loss of HCO3 – [50] and this explains lower HCO3 – concentration in mine water. It indicates that mine waters are recharged by the overlying shallower Dupi Tila aquifer.
In general, chloride is a conservative component, and evaporation and mixing are considered as the main factors controlling its concentration in groundwater. Solubility of Na+ compounds is high, so Na+ remains dissolved in water in a very wide range of concentration [51]. Most of the groundwater and coal mine water samples also plot above the 1:1 equiline (Figure 6). Plot of Na versus Cl shows an excess of Na to Cl, particularly for all coal mine water samples and also for most of the groundwater samples. The excess of sodium both in groundwater and coal mine water suggests that Na+ originates from weathering of silicate minerals from the aquifer system due to rapid inflow of groundwater into deeper Gondwana aquifer facilitated by the mining activities.
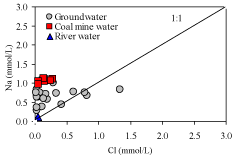
Figure 6: Bivariate plot of Na versus Cl.
Gaillardet et al. [51] stated that the Na-normalized ratios for Ca2+ and Mg2+ might have been related to each other. Accordingly, in the plot of the molar ratios of Ca/Na versus Mg/Na are shown in a log–log space in (Figure 7). Recharging water infiltrating into the Dupi Tila aquifer shows low Ca/Na and Mg/Na rations (Figure 7), and the end member having lower Na normalized ratios is that of water draining silicates. The molar Ca/Na ratio of average crustal continental rocks is close to 0.6 [52], and due to the higher solubility of Na relative to Ca, lower Ca/Na molar ratios are expected in groundwater, which are related to weathering of silicates. In (Figure 7), the observed groundwater and coal mine water with low Ca/Na molar ratios are being influenced by silicate weathering rather than carbonate dissolution [18]. Meanwhile, Stallard and Edmond [53,54] state that the molar ratio (Na+ + K+)/Cl- 1 indicates silicate (feldspar and mica) weathering. In present study, it is observed that most of the groundwater (except sample B5 and B12) and all coal mine water samples show molar ratios (Na+ + K+)/Cl– >1 (data not shown), which indicates silicate weathering. From the above discussions, it is obvious that rainfall infiltrates into the shallow Dupi Tila aquifers through the ground and hydrogeochemical reactions take place for the formation of Ca-Mg-HCO3 type water. The development of roadways within the coal mine creating voids facilitate rapid entrance of water into the coal mine through the overlying Dupi Tila and Gondwana aquifers. The initial Ca–Mg–HCO3 type water tends to change into Na-Ca- HCO3 type water due to preferentially silicate weathering, which helps to add Na+ within the solution in increase with the length of the flow path and thus the coal mine water tends to be Na–Ca–HCO3 type water due to silicate weathering giving rise to excess Na+ with high pH.
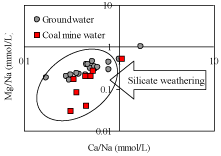
Figure 7: Molar ratio bivariate plot of Na-normalized Ca and Mg.
The Local Meteoric Water Line (LMWL) proposed by Majumder et al. [12] runs closely parallel to the GMWL (Figure 8). The entire groundwater, mine water and river water data set plotted along and/or close to the LMWL and GMWL, producing a regression line defined by d2 = 7.4d18O + 4.4 (line not shown in Figure 8) with a regression coefficient (R2) of 0.94 that suggests a meteoric origin [55,56] with insignificant localized evaporation effect. The alignment of isotopic composition close to the GMWL and LMWL demonstrates a precipitation origin of groundwater in the studied area [57]. Except sample B2, all the groundwater and mine water d18O values show narrow range (–5.4‰ to –4‰) along the LMWL (Figure 8), which suggests that these waters have mixed sufficiently to homogenize variations in the isotopic composition of recharge water. The average d18O value (–4.7‰) for the groundwater is similar to the mine water average d18O value (–4.7‰), which indicates that the groundwater percolates in to the underlying coal seam bearing Gondwana aquifers without changing infiltrating groundwater d18O values. The groundwater sample B2 with slightly enriched d18O value (–3.4‰) indicates minor evaporation effect prior to infiltration. The river water B20 d18O value (–9.3‰) is highly depleted and the extremely negative value reveals the high content of glacier and snowmelt water, which is typical for a high-altitude river. Meanwhile, the river water d18O value (-9.3‰) of the study area is similar to the d18O value for Tista river water [18], which also reflects that the substantial amounts of monsoon rainfall in the Himalayan region as well as snow melt water [58] may give rise to the depleted d18O value for the river water. The highly depleted isotopic signatures of the observed river water reveals that the aquifers in the study area may not be recharged by the river water and thus the groundwater has a local origin of recharge (i.e., rainfalls).
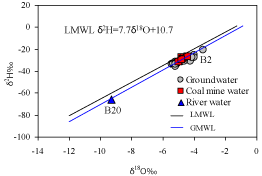
Figure 1: d-plot for d18O and d2H compositions of the groundwater, coal mine
water, and river water
Except groundwater sample B1, all the analysed water sample 3H content was less than 0.7 TU. The low tritium content of shallow groundwater and coal mine water indicate relatively large travel times in the overlying Madhupur clay zone (several years to tens of years) which may control the recharge pattern of study area, resulting in the loss of tritium by radioactive decay before reaching the aquifer. As a consequence, the groundwater and mine water with a tritium value <1 TU may be considered “old” water recharged prior to 1952 while higher values that is the B1 well water would represent “new” water being wholly or partially recharged since 1952 [59].
Conclusion
The hydrochemical and isotopic data of collected groundwater, mine water, and river water show distinct evidence of the origin of Barapukuria coal mine inflow water. EC, pH, Temperature, solute concentrations (Na+, Cl–, Ca2+, Mg2+, SO4 2–, and HCO3 –), stable isotope ratios (hydrogen and oxygen), and 3H concentrations were used successfully to identify the origin of Barapukuria coal mine inflow water. The long migration path of the infiltrating groundwater facilitates dissolution of mineral salts along the fractured Gondwana sandstone which may cause higher EC values and the coal mine water high EC values indicate a good hydraulic connectivity between the most fractured coal seam bearing Gondwana aquifer and the overlying Dupi Tila aquifers. The acidic to neutral groundwater pH values imply an open system unconfined aquifer all over the study area. But the alkaline pH values for coal mine inflow water suggest decline in partial pressure of CO2 levels within the Gondwana aquifers and indicates lack of oxygen in the deeper Gondwana aquifers which could contribute to a more alkaline pH for the inflowing coal mine water. Besides, the coal mine water higher temperature than that of groundwater temperature implies that low temperature Dupi Tila aquifer water percolates into the deeper Gondwana aquifers attaining high temperatures within the coal seams which cause high heat flow in the coal mine. Both groundwater and coal mine inflow water are dominantly of Na–Ca–HCO3 and Ca–Na–HCO3 type water and this phenomenon indicates potential connectivity between overlying Dupi Tila and underlying coal seam bearing Gondwana aquifers causing rapid inflow into the coal mine tunnel without changing chemical characteristics of recharging water. Besides, low mineralized Ca–Mg–HCO3 type water observed in both groundwater and coal mine inflow water also indicates infiltration of shallow groundwater into the deeper coal seam bearing Gondwana aquifers, which helps to increase inflow of mine water.
The relatively high Na concentration of coal mine water indicates that the mine water is recharged from overlying shallow groundwater aquifers moving slowly downward through the Gondwana aquifers promoting dilution of Na-rich minerals. Meanwhile, slightly higher Ca2+ concentration of coal mine water indicates insufficient base exchange reactions of surface water inflowing into the coal mine and thus Ca2+ does not adsorbed enough onto clays within the sediments in exchange for Na, and the insignificant cation exchange implies relatively short travel path of recharging water as well as short residence time. Strong positive correlation between Ca2+ and HCO3 – indicates that the groundwater in Dupi Tila aquifer moves downward through the Gondwana aquifer with minor dissolution of calcite giving rise to low Ca+ concentration due to relatively short residence times of the inflowing mine water within the aquifer systems. Compared to observed groundwater, relatively low HCO3 – concentrations of coal mine water attributes lack of CO2 present (confined aquifer) resulting loss of HCO3 – and it indicates that the coal mine inflow waters are mainly recharged by the overlying shallower Dupi Tila aquifer.
The clustering of groundwater, mine water and river water samples close to the LMWL indicates a common origin for aforesaid water samples, and provides compelling evidence that the observed groundwater and mine water are derived from local rainfalls with insignificant localized evaporation effect. Besides, the similarity between the average d18O composition of groundwater and the mine water indicates that the groundwater percolates in to the underlying coal seam bearing Gondwana aquifers without changing infiltrating groundwater d18O compositions. The low tritium content of shallow groundwater and coal mine water indicates relatively large travel times in the overlying Madhupur clay zone. The groundwater and mine water with a tritium value <1 TU could be considered as old water recharged prior to 1952. Finally, it can be concluded that the origin of Barapukuria coal mine inflow water is from the Dupi Tila aquifer recharged by local rainfalls which have been recharged within the aquifers prior to 1952.
Acknowledgement
The first author would like to acknowledge the Ministry of Education, Culture, Sports Science and Technology (MEXT), Japan for providing funds for Doctor of Science research during 2005 to 2008. The authors are thankful to the Barapukuria Coal Mine authority for their support during mine water sampling.
References
- Islam MR, Hayashi D. Geology and coal bed methane resource potential of the Gondwana Barapukuria Coal Basin, Dinajpur, Bangladesh. Intl J Coal Geol. 2008; 75: 127-143.
- Kibria MG, Quamruzzaman C, Woobaid Ullah ASM, Kabir AKMF. Effect of longwall mining on groundwater for underground coal extraction in Barapukuria, Bangladesh. J Mines, Metals & Fuels IP / DG / M-MA / F/. 2012; 18.5.12: 60-66.
- Howladar MF, Deb PK, Muzemder ATMSH, Miah MI. An Assessment of the Underground Roadway Water Quality for Irrigation Use around the Barapukuria Coal Mining Industry, Dinajpur, Bangladesh. International Conference on Mechanical, Industrial and Energy Engineering. 2014.
- Halim MA, Majumder RK, Zaman MN, Hossain S, Rasul MG, Sasaki K. Mobility and impact of trace metals in Barapukuria coal mining area, Northwest Bangladesh. Arab J Geosci. 2013; 6: 4593-4605.
- Halim MA, Majumder RK, Zaman MN. Paddy soil heavy metal contamination and uptake in rice plants from the adjacent area of Barapukuria coal mine, northwest Bangladesh. Arab J Geosci. 2014; 8: 3391-3401.
- Parizi HS, Samani N. Environmental Isotope Investigation of Groundwater in the Sarcheshmeh Copper Mine Area, Iran. Mine Water Environ. 2014; 33: 97-109.
- Fantong WY, Satake H, Aka FT, Ayonghe SN, Asai K, Mandal AK, Ako AA. Hydrochemical and isotopic evidence of recharge apparent age and flow direction of groundwater in the Mayo Tsanaga River Basin Cameroon: bearings on contamination. Environ Earth Sci. 2010; 60: 107-120.
- Matter JM, Waber HN, Loew S, Matter A. Recharge areas and geochemical evolution of groundwater in an alluvial aquifer system in the Sultanate of Oman. Hydrogeol J. 2005; 14:203-224.
- Morton KL, Mekerk FA. A phased approach to mine dewatering. Mine Water Environ. 1993; 12: 27-33.
- Girard P, Hillaire-Marcel C, Oga MS. Determining the recharge mode of sahelian aquifers using water isotopes. J Hydrol. 1997; 197:189-202.
- Geirnaert W, Groenand M, Van der Sommen J. Isotope studies as a final stage in groundwater investigations on the African shield. In: Challenges in African hydrology and water resources. IAHS publication, Harare. 1984; 144: 141-153.
- Majumder RK, Halim MA, Saha BB, Ikawa R, Nakamura T, Kagabu M, Shimada J. Groundwater flow system in Bengal Delta, Bangladesh revealed by environmental isotopes. Environ Earth Sci. 2011; 64: 1343-1352.
- Fritz P, Hennings CS, Suzuki O, Salari E. Isotope hydrology in northern Chile. In: International Atomic Energy Agency (ed) Isotope hydrology. Vienna, Austria. 1979; 525-544.
- Das BK, Kakar YP, Moser H, Stichler W. Deuterium and oxygen-18 studies in groundwater of the Dehli area. India J Hydrol. 1988; 98: 133-146.
- Leontiadias IL, Payne BR, Christodoulou T. Isotope hydrology of the Aghios Nikolaos area of Crete, Greece. J Hydrol. 1988; 98: 121-132.
- Fontes JC. Environmental isotopes in groundwater hydrology. Fritz P, Fontes JC, editors. In: Handbook of environmental isotope geochemistry. Elsevier, Amsterdam. 1980; 75-140.
- Gat JR. The isotopes of hydrogen and oxygen in precipitation. Fritz P, Fontes JC, editors. In: Handbook of environmental isotope geochemistry. Elsevier, New York. 1980.
- Majumder RK. Groundwater flow systems of Bengal Delta, Bangladesh, revealed by environmental isotopes and hydrochemistry (unpublished D.Sc. Thesis, Kumamoto University, Japan). 2008.
- Dassi L, Zouari K, Seiler KP, Faye S, Kamel S. Flow exchange between the deep and shallow groundwaters in the Sbeitla synclinal basin (Tunisia): an isotopic approach. Environ Geol. 2004; 47: 501-511.
- Rahman A. Geology of Madhyapara Area, Dinajpur District, Bangladesh. Rec. Geol. Surv. Bengal. 1987; 5.
- Khan FH. Geology of Bangladesh, University Press Limited, Dhaka, Bangladesh. 1991.
- Wardell A. Techno-economic feasibility study, Barapukuria coal project, Dinajpur District, Bangladesh. 1991; 1.
- Bakr AM, Rahman QMA, Islam MM. Barapukuria coal deposit, Parbatipur Upazilla, Dinajpur District. Records of the Geological Survey of Bangladesh. 1986; 2: 35.
- Uddin MN, Islam S. Gondwana basins and their coal resources in Bangladesh (Abst.). First South Asia Geol Congr Pakistan. 1992; 30.
- CMC. Preliminary Geology and Exploration Report of Barapukuria Coal Mine, Bangladesh. 1994.
- Majumder RK, Halim MA, Shimada J, Saha BB, Zahid A, Hasan MQ, et al. Hydrochemistry and isotopic studies to identify Ganges River and riverbank groundwater interaction, southern Bangladesh. Arab J Geosci. 2013; 6: 4585-4591.
- Halim MA, Majumder RK, Rasul G, Hirosiro Y, Sasaki K, Shimada J, Jinno K. Geochemical Evaluation of Arsenic and Manganese in Shallow Groundwater and Core Sediment in Singair Upazila, Central Bangladesh. Arab J Sci Engin. 2014; 39: 5585-5601.
- Coplen TB. New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data. Geoch et Cosmoch Acta. 1996; 60: 3359-3360.
- Craig H. Isotopic Variations in Meteoric Waters. Science. 1961; 133: 1702-1703.
- Solomon DK, Cook PG. 3H and 3He. In: Cook P, Herczeg AL (eds). Environmental tracers in subsurface hydrology. Kluwer Academic, Boston. 1999; 397-424.
- Yokoyama T. A temperature analysis of groundwater flow system in the upper part of the Ashigara plain, Tracers in Hydrology (Proc of the Yokohama Symp, July 1993). IAHS. 1993; 215.
- Piper AM. A graphic procedure in geochemical interpretation of water analysis, American Geophysics Union. Transactions. 1944; 25: 914-923.
- Soulsby C, Chen M, Ferrier RC, Helliwell C, Jenkins A, Harriman R. Hydrogeochemistry of shallow groundwater in an upland Scottish catchment. Hydrol Proce. 1998; 12: 1111–1127.
- Aston TRC. Technical Note Tritium Dating of Water Inflows at the Donkin-Morien Project, Nova Scotia. Intl J of Mine Water. 1985; 4: 41-52.
- Appelo CAJ, Postma D. Geochemistry, groundwater and pollution, 2nd edition. AA Balkema, Rotterdam, 2005.
- James ER, Manga M, Rose TP, Hudson GB. The use of temperature and the isotopes of O, H, C, and noble gases to determine the pattern and spatial extent of groundwater flow. J Hydrol. 2000; 237: 100-112.
- Taniguchi M. Evaluation of vertical groundwater fluxes and thermal properties of aquifers based on transient temperature-depth profiles. Water Res. 1993; 29: 2021-2026.
- Kabir SM. Subsurface temperature and geothermal gradient in Bangladesh. Dhaka University, Department of Geology, MS thesis (unpubl). 2008.
- Vermeulen PD, Burger M, Wyk Avan, Lukas E. Using Environmental Isotopes in a Coal Mine and a Gold Mine to Determine Groundwater Interaction. Mine Water Environ. 2014; 33: 15-23.
- Eby GN. Principles of environmental geochemistry. Thomson Brooks/Cole, Pacific Grove. 2004.
- Hodgson FDI, Krantz RM. Investigation into the groundwater quality deterioration in the Olifants River catchment above the Loskop dam with specialised investigation into the Witbank dam sub-catchment. Inst for Groundwater Studies, Univ of the Free State, Bloemfontein. 1995.
- Halim MA, Majumder RK, Nessa SA, Hiroshiro Y, Uddin MJ, Shimada J, et al. Hydrogeochemistry and arsenic contamination of groundwater in the Ganges Delta Plain, Bangladesh. J Hazard Mater. 2009; 164: 1335-1345.
- Halim MA, Majumder RK, Nessa SA, Hiroshiro Y, Sasaki K, Saha BB, et al. Evaluation of processes controlling the geochemical constituents in deep groundwater in Bangladesh: Spatial variability on arsenic and boron enrichment. J Hazard Mater. 2010, 180: 50-62.
- Vermeulen PD, Usher BH. Sulphate generation in South Africa underground and opencast collieries. Environ Geol. 2006; 49: 552-569.
- Mukherjee A, Fryar AE. Deeper groundwater chemistry and geochemical modeling of the arsenic affected western Bengal basin, West Bengal, India. Appl. Geochem. 2008; 23: 863-894.
- Mapoma HWT, Xie X, Pi K, Liua Y, Zhua Y. Understanding arsenic mobilization using reactive transport modeling of groundwater hydrochemistry in the Datong basin study plot, China. Environ. Sci. Processes Impacts. 2016; 18: 371.
- Drever JI. The geochemistry of natural waters. Prentice Hall, Englewood Cliffs. 1988.
- Kinniburgh DG, Smedley PL. Arsenic Contamination of Groundwater in Bangladesh, vol. 2. Technical Report WC/00/19, British Geological Survey. 2001; 267.
- Ueno N. Social scientific village research at Samta. In: Abstract of the 5th Forum on Arsenic Contamination of Groundwater in Asia, Yokohama. 2000; 27-36.
- Freeze RA, Cherry JA. Groundwater. Prentice-Hall, Englewood Cliffs. 1979; 604.
- Gaillardet J, Dupre B, Louvat P, Allegre CJ. Global silicate weathering and CO consumption rates deduced 2 from the chemistry of large rivers. Chem Geol. 1998; 159: 3-30.
- Taylor SR, McLennan SM. The Continental Crust: its Composition and Evolution. Blackwell, London. 1985; 321.
- Stallard RF, Edmond JM. Geochemistry of the Amazon 2. The influence of geology and the weathering environment on the dissolved load. J Geophy Res. 1983; 88: 9671-9688.
- Stallard RF, Edmond JM. Geochemistry of the Amazon 3. Weathering chemistry and limits to dissolved inputs. J Geophy Res. 1987; 92: 8293-8302.
- Monjerezi M, Vogt RD, Aagaard P, Gebru AG, Saka JD. Using 87Sr/86Sr, d18O and d2H isotopes along with major chemical composition to assess groundwater salinization in lower Shire valley, Malawi. Appl Geochem. 2011; 26: 2201-2214.
- Mapoma HWT, Xie X, Zhang L. Redox control on trace element geochemistry and provenance of groundwater in fractured basement of Blantyre, Malawi. J Afr Earth Sci. 2014; 100: 335-345.
- Mapoma HWT, Xie X, Zhu Y, Liu Y, Sitolo-Banda GC. Trace element geochemical evolution and groundwater origin in North Rukuru–Songwe alluvial aquifer of northern Malawi. Environ Earth Sci. 2016; 75: 877.
- Lambs L, Balakrishna K, Brunet F, Probst JL. Oxygen and hydrogen isotopic composition of major Indian rivers: a first global assessment. Hydrol Proce. 2005; 19: 3345-3355.
- Cowie R, Williams MW, Wireman M, Runkel RL. Use of Natural and Applied Tracers to Guide Targeted Remediation Efforts in an Acid Mine Drainage System, Colorado Rockies, USA. Water. 2014; 6: 745-777.